Selective targeting of microglia by quantum dots
- PMID: 22272874
- PMCID: PMC3292839
- DOI: 10.1186/1742-2094-9-22
Selective targeting of microglia by quantum dots
Abstract
Background: Microglia, the resident immune cells of the brain, have been implicated in brain injury and various neurological disorders. However, their precise roles in different pathophysiological situations remain enigmatic and may range from detrimental to protective. Targeting the delivery of biologically active compounds to microglia could help elucidate these roles and facilitate the therapeutic modulation of microglial functions in neurological diseases.
Methods: Here we employ primary cell cultures and stereotaxic injections into mouse brain to investigate the cell type specific localization of semiconductor quantum dots (QDs) in vitro and in vivo. Two potential receptors for QDs are identified using pharmacological inhibitors and neutralizing antibodies.
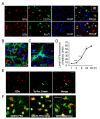
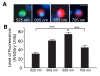
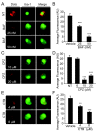
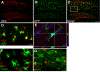
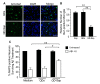
Results: In mixed primary cortical cultures, QDs were selectively taken up by microglia; this uptake was decreased by inhibitors of clathrin-dependent endocytosis, implicating the endosomal pathway as the major route of entry for QDs into microglia. Furthermore, inhibiting mannose receptors and macrophage scavenger receptors blocked the uptake of QDs by microglia, indicating that QD uptake occurs through microglia-specific receptor endocytosis. When injected into the brain, QDs were taken up primarily by microglia and with high efficiency. In primary cortical cultures, QDs conjugated to the toxin saporin depleted microglia in mixed primary cortical cultures, protecting neurons in these cultures against amyloid beta-induced neurotoxicity.
Conclusions: These findings demonstrate that QDs can be used to specifically label and modulate microglia in primary cortical cultures and in brain and may allow for the selective delivery of therapeutic agents to these cells.
Figures
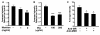


Similar articles
-
SPARC regulates microgliosis and functional recovery following cortical ischemia.J Neurosci. 2013 Mar 6;33(10):4468-81. doi: 10.1523/JNEUROSCI.3585-12.2013. J Neurosci. 2013. PMID: 23467362 Free PMC article.
-
Neonatal rat microglia derived from different brain regions have distinct activation responses.Neuron Glia Biol. 2011 Feb;7(1):5-16. doi: 10.1017/S1740925X12000154. Neuron Glia Biol. 2011. PMID: 22857737
-
An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ.J Neurosci. 2012 Jul 11;32(28):9677-89. doi: 10.1523/JNEUROSCI.4742-11.2012. J Neurosci. 2012. PMID: 22787053 Free PMC article. Clinical Trial.
-
A limited capacity for microglial repopulation in the adult brain.Glia. 2018 Nov;66(11):2385-2396. doi: 10.1002/glia.23477. Epub 2018 Oct 28. Glia. 2018. PMID: 30370589 Free PMC article.
-
Oxidative stress-induced neurotoxicity of quantum dots and influencing factors.Nanomedicine (Lond). 2024;19(11):1013-1028. doi: 10.2217/nnm-2023-0326. Epub 2024 Apr 12. Nanomedicine (Lond). 2024. PMID: 38606672 Review.
Cited by
-
Pharmacological Tools to Activate Microglia and their Possible use to Study Neural Network Patho-physiology.Curr Neuropharmacol. 2017;15(4):595-619. doi: 10.2174/1570159X14666160928151546. Curr Neuropharmacol. 2017. PMID: 27697040 Free PMC article. Review.
-
Microglia and inflammation: conspiracy, controversy or control?Cell Mol Life Sci. 2014 Oct;71(20):3969-85. doi: 10.1007/s00018-014-1670-8. Epub 2014 Jul 10. Cell Mol Life Sci. 2014. PMID: 25008043 Free PMC article. Review.
-
Targeting Beclin1 as an Adjunctive Therapy against HIV Using Mannosylated Polyethylenimine Nanoparticles.Pharmaceutics. 2021 Feb 6;13(2):223. doi: 10.3390/pharmaceutics13020223. Pharmaceutics. 2021. PMID: 33561939 Free PMC article.
-
Advances in Nanomaterials for Brain Microscopy.Nano Res. 2018 Oct;11(10):5144-5172. doi: 10.1007/s12274-018-2145-2. Epub 2018 Aug 8. Nano Res. 2018. PMID: 31105899 Free PMC article.
-
Delivery and tracking of quantum dot peptide bioconjugates in an intact developing avian brain.ACS Chem Neurosci. 2015 Mar 18;6(3):494-504. doi: 10.1021/acschemneuro.5b00022. Epub 2015 Mar 5. ACS Chem Neurosci. 2015. PMID: 25688887 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

