MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression
- PMID: 22272270
- PMCID: PMC3260191
- DOI: 10.1371/journal.pone.0030030
MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression
Abstract
Background: Early-life infection by respiratory syncytial virus (RSV) is associated with aberrant expression of the prototypical neurotrophin nerve growth factor (NGF) and its cognate receptors in human bronchial epithelium. However, the chain of events leading to this outcome, and its functional implications for the progression of the viral infection, has not been elucidated. This study sought to test the hypothesis that RSV infection modulates neurotrophic pathways in human airways by silencing the expression of specific microRNAs (miRNAs), and that this effect favors viral growth by interfering with programmed death of infected cells.
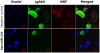
Methodology: Human bronchial epithelial cells infected with green fluorescent protein-expressing RSV (rgRSV) were screened with multiplex qPCR arrays, and miRNAs significantly affected by the virus were analyzed for homology with mRNAs encoding neurotrophic factors or receptors. Mimic sequences of selected miRNAs were transfected into non-infected bronchial cells to confirm the role of each of them in regulating neurotrophins expression at the gene and protein level, and to study their influence on cell cycle and viral replication.
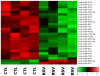
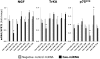
Principal findings: RSV caused downregulation of 24 miRNAs and upregulation of 2 (p<0.01). Homology analysis of microarray data revealed that 6 of those miRNAs exhibited a high degree of complementarity to NGF and/or one of its cognate receptors TrKA and p75(NTR). Among the selected miRNAs, miR-221 was significantly downregulated by RSV and its transfection in bronchial epithelial cells maximally inhibited gene and protein expression of NGF and TrKA, increased apoptotic cell death, and reduced viral replication and infectivity.
Conclusions/significance: Our data suggest that RSV upregulates the NGF-TrKA axis in human airways by silencing miR-221 expression, and this favors viral replication by interfering with the apoptotic death of infected cells. Consequently, the targeted delivery of exogenous miRNAs to the airways may provide a new strategy for future antiviral therapies based on RNA interference.
Conflict of interest statement
Figures
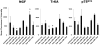



Similar articles
-
NGF is an essential survival factor for bronchial epithelial cells during respiratory syncytial virus infection.PLoS One. 2009 Jul 31;4(7):e6444. doi: 10.1371/journal.pone.0006444. PLoS One. 2009. PMID: 19649262 Free PMC article.
-
Nanoparticles increase human bronchial epithelial cell susceptibility to respiratory syncytial virus infection via nerve growth factor-induced autophagy.Physiol Rep. 2017 Jul;5(13):e13344. doi: 10.14814/phy2.13344. Epub 2017 Jul 11. Physiol Rep. 2017. PMID: 28701524 Free PMC article.
-
Nerve growth factor modulates human rhinovirus infection in airway epithelial cells by controlling ICAM-1 expression.Am J Physiol Lung Cell Mol Physiol. 2012 May 15;302(10):L1057-66. doi: 10.1152/ajplung.00365.2011. Epub 2012 Mar 16. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22427528 Free PMC article.
-
Role of Nerve Growth Factor (NGF) and miRNAs in Epithelial Ovarian Cancer.Int J Mol Sci. 2017 Feb 26;18(3):507. doi: 10.3390/ijms18030507. Int J Mol Sci. 2017. PMID: 28245631 Free PMC article. Review.
-
Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection.Pediatr Infect Dis J. 2003 Feb;22(2 Suppl):S66-74; discussion S74-5. doi: 10.1097/01.inf.0000053888.67311.1d. Pediatr Infect Dis J. 2003. PMID: 12671455 Review.
Cited by
-
MicroRNAs in Asthma and Respiratory Infections: Identifying Common Pathways.Allergy Asthma Immunol Res. 2020 Jan;12(1):4-23. doi: 10.4168/aair.2020.12.1.4. Allergy Asthma Immunol Res. 2020. PMID: 31743961 Free PMC article. Review.
-
The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.Int Immunopharmacol. 2021 Jan;90:107204. doi: 10.1016/j.intimp.2020.107204. Epub 2020 Nov 13. Int Immunopharmacol. 2021. PMID: 33221169 Free PMC article. Review.
-
Respiratory syncytial virus and asthma: speed-dating or long-term relationship?Curr Opin Pediatr. 2013 Jun;25(3):344-9. doi: 10.1097/MOP.0b013e328360bd2e. Curr Opin Pediatr. 2013. PMID: 23657245 Free PMC article. Review.
-
Apoptosis in pneumovirus infection.Viruses. 2013 Jan 23;5(1):406-22. doi: 10.3390/v5010406. Viruses. 2013. PMID: 23344499 Free PMC article. Review.
-
MicroRNA Regulation of RNA Virus Replication and Pathogenesis.Trends Mol Med. 2017 Jan;23(1):80-93. doi: 10.1016/j.molmed.2016.11.003. Epub 2016 Dec 16. Trends Mol Med. 2017. PMID: 27989642 Free PMC article. Review.
References
-
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: Past, present, and future. Pediatr Pulmonol. 2011;46:324–347. - PubMed
-
- Scuri M, Samsell L, Piedimonte G. The role of neurotrophins in inflammation and allergy. Inflamm Allergy Drug Targets. 2010;9:173–180. - PubMed
-
- Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, et al. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172:233–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

