The human immunodeficiency virus-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the proliferation of multiple myeloma cells in vitro and in vivo
- PMID: 22271897
- PMCID: PMC3396684
- DOI: 10.3324/haematol.2011.049981
The human immunodeficiency virus-1 protease inhibitor nelfinavir impairs proteasome activity and inhibits the proliferation of multiple myeloma cells in vitro and in vivo
Abstract
Background: Multiple myeloma is characterized by the accumulation of tumor plasma cells in the bone marrow. Despite therapeutic improvements brought by proteasome inhibitors such as bortezomib, myeloma remains an incurable disease. In a variety of human cancers, human immunodeficiency virus protease inhibitors (e.g. nelfinavir) effectively inhibit tumor progression, but their impact on myeloma is unknown. We assessed the in vitro and in vivo effects of nelfinavir on multiple myeloma.
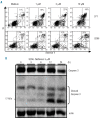
Design and methods: The effects of nelfinavir (1-10 μM) on proteasome activity, proliferation and viability of myeloma cell lines and plasma cells from patients were assessed by measuring PERK, AKT, STAT3 and ERK1/2 phosphorylation and CHOP expression with immunoblotting or flow cytometry. The in vivo effect was assessed in NOD/SCID mice injected with luciferase expressing human myeloma cell lines and treated with nelfinavir at a dose of 75 mg/kg/day. Tumor progression was evaluated using a bioluminescent system.
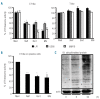
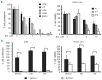
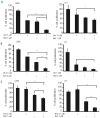
Results: Nelfinavir inhibited 26S chymotrypsin-like proteasome activity, impaired proliferation and triggered apoptosis of the myeloma cell lines and fresh plasma cells. It activated the pro-apoptotic unfolded protein response pathway by inducing PERK phosphorylation and CHOP expression. Cell death triggered by nelfinavir treatment correlated with decreased phosphorylation of AKT, STAT3 and ERK1/2. Nelfinavir enhanced the anti-proliferative activity of bortezomib, dexamethasone and histone deacetylase inhibitors and delayed tumor growth in a myeloma mouse model.
Conclusions: These results suggest that nelfinavir, used at a pharmacological dosage, alone or in combination, may be useful in the treatment of myeloma. Our data provide a preclinical basis for clinical trials using nelfinavir in patients with myeloma.
Figures


Similar articles
-
In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells.Clin Cancer Res. 2011 Aug 15;17(16):5311-21. doi: 10.1158/1078-0432.CCR-11-0476. Epub 2011 Jun 30. Clin Cancer Res. 2011. PMID: 21724551 Free PMC article.
-
Preventing the autophagic survival response by inhibition of calpain enhances the cytotoxic activity of bortezomib in vitro and in vivo.Cancer Chemother Pharmacol. 2013 Jun;71(6):1567-76. doi: 10.1007/s00280-013-2156-3. Epub 2013 Apr 10. Cancer Chemother Pharmacol. 2013. PMID: 23572175 Free PMC article.
-
Effect of noncompetitive proteasome inhibition on bortezomib resistance.J Natl Cancer Inst. 2010 Jul 21;102(14):1069-82. doi: 10.1093/jnci/djq198. Epub 2010 May 26. J Natl Cancer Inst. 2010. PMID: 20505154
-
Combined proteasome and histone deacetylase inhibition: A promising synergy for patients with relapsed/refractory multiple myeloma.Leuk Res. 2010 Sep;34(9):1111-8. doi: 10.1016/j.leukres.2010.04.001. Epub 2010 May 15. Leuk Res. 2010. PMID: 20472288 Review.
-
Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma.Mol Cancer Ther. 2011 Nov;10(11):2034-42. doi: 10.1158/1535-7163.MCT-11-0433. Mol Cancer Ther. 2011. PMID: 22072815 Free PMC article. Review.
Cited by
-
Marketed nonsteroidal anti-inflammatory agents, antihypertensives, and human immunodeficiency virus protease inhibitors: as-yet-unused weapons of the oncologists' arsenal.Ther Clin Risk Manag. 2015 May 18;11:807-19. doi: 10.2147/TCRM.S82049. eCollection 2015. Ther Clin Risk Manag. 2015. PMID: 26056460 Free PMC article. Review.
-
Targeting chromosome 12q amplification in relapsed glioblastoma: the use of computational biological modeling to identify effective therapy-a case report.Ann Transl Med. 2022 Dec;10(23):1289. doi: 10.21037/atm-2022-62. Ann Transl Med. 2022. PMID: 36618786 Free PMC article.
-
Nelfinavir Inhibits the TCF11/Nrf1-Mediated Proteasome Recovery Pathway in Multiple Myeloma.Cancers (Basel). 2020 Apr 25;12(5):1065. doi: 10.3390/cancers12051065. Cancers (Basel). 2020. PMID: 32344880 Free PMC article.
-
Insights into the broad cellular effects of nelfinavir and the HIV protease inhibitors supporting their role in cancer treatment and prevention.Curr Opin Oncol. 2013 Sep;25(5):495-502. doi: 10.1097/CCO.0b013e328363dfee. Curr Opin Oncol. 2013. PMID: 23872785 Free PMC article. Review.
-
A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care.Oncotarget. 2013 Apr;4(4):502-30. doi: 10.18632/oncotarget.969. Oncotarget. 2013. PMID: 23594434 Free PMC article. Review.
References
-
- Bruno B, Giaccone L, Rotta M, Anderson K, Boccadoro M. Novel targeted drugs for the treatment of multiple myeloma: from bench to bedside. Leukemia. 2005;19(10):1729–38. - PubMed
-
- Brenner H, Gondos A, Pulte D. Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood. 2008;111(5):2521–6. - PubMed
-
- Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12(6):664–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

