A change in nuclear pore complex composition regulates cell differentiation
- PMID: 22264802
- PMCID: PMC3288503
- DOI: 10.1016/j.devcel.2011.11.021
A change in nuclear pore complex composition regulates cell differentiation
Abstract
Nuclear pore complexes (NPCs) are built from ∼30 different proteins called nucleoporins or Nups. Previous studies have shown that several Nups exhibit cell-type-specific expression and that mutations in NPC components result in tissue-specific diseases. Here we show that a specific change in NPC composition is required for both myogenic and neuronal differentiation. The transmembrane nucleoporin Nup210 is absent in proliferating myoblasts and embryonic stem cells (ESCs) but becomes expressed and incorporated into NPCs during cell differentiation. Preventing Nup210 production by RNAi blocks myogenesis and the differentiation of ESCs into neuroprogenitors. We found that the addition of Nup210 to NPCs does not affect nuclear transport but is required for the induction of genes that are essential for cell differentiation. Our results identify a single change in NPC composition as an essential step in cell differentiation and establish a role for Nup210 in gene expression regulation and cell fate determination.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

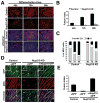


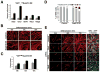
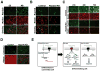
Comment in
-
Development: NUP210 takes cell fate decisions.Nat Rev Mol Cell Biol. 2012 Feb 1;13(3):140. doi: 10.1038/nrm3285. Nat Rev Mol Cell Biol. 2012. PMID: 22293614 No abstract available.
-
Differentiation: Nuclear pores at the core.Nat Rev Genet. 2012 Feb 7;13(3):151. doi: 10.1038/nrg3179. Nat Rev Genet. 2012. PMID: 22310893 No abstract available.
Similar articles
-
Nup358, a nucleoporin, functions as a key determinant of the nuclear pore complex structure remodeling during skeletal myogenesis.FEBS J. 2011 Feb;278(4):610-21. doi: 10.1111/j.1742-4658.2010.07982.x. Epub 2011 Jan 4. FEBS J. 2011. PMID: 21205196
-
Nuclear Pores Regulate Muscle Development and Maintenance by Assembling a Localized Mef2C Complex.Dev Cell. 2017 Jun 5;41(5):540-554.e7. doi: 10.1016/j.devcel.2017.05.007. Dev Cell. 2017. PMID: 28586646 Free PMC article.
-
Changes in Nup62 content affect contact-induced differentiation of cultured myoblasts.Differentiation. 2020 Jul-Aug;114:27-35. doi: 10.1016/j.diff.2020.05.001. Epub 2020 May 19. Differentiation. 2020. PMID: 32554220
-
Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions.Nat Rev Mol Cell Biol. 2012 Nov;13(11):687-99. doi: 10.1038/nrm3461. Nat Rev Mol Cell Biol. 2012. PMID: 23090414 Review.
-
The nuclear pore complex: understanding its function through structural insight.Nat Rev Mol Cell Biol. 2017 Feb;18(2):73-89. doi: 10.1038/nrm.2016.147. Epub 2016 Dec 21. Nat Rev Mol Cell Biol. 2017. PMID: 27999437 Review.
Cited by
-
Red5 and three nuclear pore components are essential for efficient suppression of specific mRNAs during vegetative growth of fission yeast.Nucleic Acids Res. 2013 Jul;41(13):6674-86. doi: 10.1093/nar/gkt363. Epub 2013 May 8. Nucleic Acids Res. 2013. PMID: 23658229 Free PMC article.
-
Tissue-Specific Gene Repositioning by Muscle Nuclear Membrane Proteins Enhances Repression of Critical Developmental Genes during Myogenesis.Mol Cell. 2016 Jun 16;62(6):834-847. doi: 10.1016/j.molcel.2016.04.035. Epub 2016 Jun 2. Mol Cell. 2016. PMID: 27264872 Free PMC article.
-
Development: NUP210 takes cell fate decisions.Nat Rev Mol Cell Biol. 2012 Feb 1;13(3):140. doi: 10.1038/nrm3285. Nat Rev Mol Cell Biol. 2012. PMID: 22293614 No abstract available.
-
Congenital Heart Disease Genetics Uncovers Context-Dependent Organization and Function of Nucleoporins at Cilia.Dev Cell. 2016 Sep 12;38(5):478-92. doi: 10.1016/j.devcel.2016.08.002. Epub 2016 Sep 1. Dev Cell. 2016. PMID: 27593162 Free PMC article.
-
NPHP proteins are binding partners of nucleoporins at the base of the primary cilium.PLoS One. 2019 Sep 25;14(9):e0222924. doi: 10.1371/journal.pone.0222924. eCollection 2019. PLoS One. 2019. PMID: 31553752 Free PMC article.
References
-
- Antonin W, Franz C, Haselmann U, Antony C, Mattaj IW. The Integral Membrane Nucleoporin pom121 Functionally Links Nuclear Pore Complex Assembly and Nuclear Envelope Formation. Mol Cell. 2005;17:83–92. - PubMed
-
- Bain G, Mansergh FC, Wride MA, Hance JE, Isogawa A, Rancourt SL, Ray WJ, Yoshimura Y, Tsuzuki T, Gottlieb DI, et al. ES cell neural differentiation reveals a substantial number of novel ESTs. Funct Integr Genomics. 2000;1:127–139. - PubMed
-
- Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, Walsh CA, Magal N, Taub E, Drasinover V, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol. 2006;60:214–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

