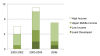Trends in compulsory licensing of pharmaceuticals since the Doha Declaration: a database analysis
- PMID: 22253577
- PMCID: PMC3254665
- DOI: 10.1371/journal.pmed.1001154
Trends in compulsory licensing of pharmaceuticals since the Doha Declaration: a database analysis
Abstract
Background: It is now a decade since the World Trade Organization (WTO) adopted the "Declaration on the TRIPS Agreement and Public Health" at its 4th Ministerial Conference in Doha. Many anticipated that these actions would lead nations to claim compulsory licenses (CLs) for pharmaceutical products with greater regularity. A CL is the use of a patented innovation that has been licensed by a state without the permission of the patent title holder. Skeptics doubted that many CLs would occur, given political pressure against CL activity and continued health system weakness in poor countries. The subsequent decade has seen little systematic assessment of the Doha Declaration's impact.
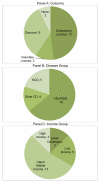
Methods and findings: We assembled a database of all episodes in which a CL was publically entertained or announced by a WTO member state since 1995. Broad searches of CL activity were conducted using media, academic, and legal databases, yielding 34 potential CL episodes in 26 countries. Country- and product-specific searches were used to verify government participation, resulting in a final database of 24 verified CLs in 17 nations. We coded CL episodes in terms of outcome, national income, and disease group over three distinct periods of CL activity. Most CL episodes occurred between 2003 and 2005, involved drugs for HIV/AIDS, and occurred in upper-middle-income countries (UMICs). Aside from HIV/AIDS, few CL episodes involved communicable disease, and none occurred in least-developed or low-income countries.
Conclusions: Given skepticism about the Doha Declaration's likely impact, we note the relatively high occurrence of CLs, yet CL activity has diminished markedly since 2006. While UMICs have high CL activity and strong incentives to use CLs compared to other countries, we note considerable countervailing pressures against CL use even in UMICs. We conclude that there is a low probability of continued CL activity. We highlight the need for further systematic evaluation of global health governance actions. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Medicine procurement and the use of flexibilities in the Agreement on Trade-Related Aspects of Intellectual Property Rights, 2001-2016.Bull World Health Organ. 2018 Mar 1;96(3):185-193. doi: 10.2471/BLT.17.199364. Epub 2018 Feb 5. Bull World Health Organ. 2018. PMID: 29531417 Free PMC article.
-
Compulsory Licensing of Pharmaceuticals in High-Income Countries: A Comparative Analysis.Milbank Q. 2022 Mar;100(1):284-313. doi: 10.1111/1468-0009.12557. Epub 2022 Mar 7. Milbank Q. 2022. PMID: 35257415 Free PMC article.
-
Compulsory licensing of pharmaceuticals reconsidered: Current situation and implications for access to medicines.Glob Public Health. 2018 Oct;13(10):1430-1440. doi: 10.1080/17441692.2017.1407811. Epub 2017 Nov 28. Glob Public Health. 2018. PMID: 29183271 Review.
-
AIDS, essential medicines, and compulsory licensing.J Int Assoc Physicians AIDS Care. 1999 Apr;5(4):24-5. J Int Assoc Physicians AIDS Care. 1999. PMID: 11367046
-
Driving a decade of change: HIV/AIDS, patents and access to medicines for all.J Int AIDS Soc. 2011 Mar 27;14:15. doi: 10.1186/1758-2652-14-15. J Int AIDS Soc. 2011. PMID: 21439089 Free PMC article. Review.
Cited by
-
Medicine procurement and the use of flexibilities in the Agreement on Trade-Related Aspects of Intellectual Property Rights, 2001-2016.Bull World Health Organ. 2018 Mar 1;96(3):185-193. doi: 10.2471/BLT.17.199364. Epub 2018 Feb 5. Bull World Health Organ. 2018. PMID: 29531417 Free PMC article.
-
USMCA (NAFTA 2.0): tightening the constraints on the right to regulate for public health.Global Health. 2019 May 14;15(1):35. doi: 10.1186/s12992-019-0476-8. Global Health. 2019. PMID: 31088499 Free PMC article. Review.
-
The role of patent waivers and compulsory licensing in facilitating access to COVID-19 vaccines: Findings from a survey among healthcare practitioners in Nigeria.PLOS Glob Public Health. 2022 Jul 7;2(7):e0000683. doi: 10.1371/journal.pgph.0000683. eCollection 2022. PLOS Glob Public Health. 2022. PMID: 36962435 Free PMC article.
-
Importance of the intellectual property system in attempting compulsory licensing of pharmaceuticals: a cross-sectional analysis.Global Health. 2019 Jun 27;15(1):42. doi: 10.1186/s12992-019-0485-7. Global Health. 2019. PMID: 31248441 Free PMC article.
-
Global pharmaceutical regulation: the challenge of integration for developing states.Global Health. 2016 Dec 20;12(1):85. doi: 10.1186/s12992-016-0208-2. Global Health. 2016. PMID: 27998293 Free PMC article.
References
-
- World Trade Organization. Declaration on the TRIPS agreement and public health. 2001 November 20. Available: http://www.wto.org/english/thewto_e/minist_e/min01_e/mindecl_trips_e.htm. Accessed 3 September 2011.
-
- World Trade Organization. Implementation of paragraph 6 of the Doha Declaration on the TRIPS Agreement and public health. 2003 September 1. Available: http://www.wto.org/english/tratop_e/trips_e/implem_para6_e.htm. Accessed 3 September 2011.
-
- Banta D. Public health triumphs at WTO Conference. JAMA. 2001;286:2655–2656. - PubMed
-
- Kanth R. WTO drug deal to spark competition. Business Times Singapore; 2003 September 2.
-
- Attaran A. How do patents and economic policies affect access to essential medicines in developing countries? Health Aff. 2004;23:155–166. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources