Development of a novel method for analyzing collagen O-glycosylations by hydrazide chemistry
- PMID: 22247541
- PMCID: PMC3433922
- DOI: 10.1074/mcp.M111.010397
Development of a novel method for analyzing collagen O-glycosylations by hydrazide chemistry
Abstract
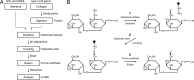
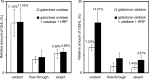
In recent years, glycopeptide purification by hydrazide chemistry has become popular in structural studies of glycoconjugates; however, applications of this method have been almost completely restricted to analysis of the N-glycoproteome. Here we report a novel method for analyzing O-glycosylations unique to collagen, which are attached to hydroxylysine and include galactosyl-hydroxylysine and glucosyl-galactosyl-hydroxylysine. We established a hydrazide chemistry-based glycopeptide purification method using (1) galactose oxidase to introduce an aldehyde into glycopeptides and (2) formic acid with heating to elute the bound glycopeptides by cleaving the hydrazone bond. This method allows not only identification of O-glycosylation sites in collagen but also concurrent discrimination of two types of carbohydrate substitutions. In bovine type I and type II collagens, galactosyl-hydroxylysine /glucosyl-galactosyl-hydroxylysine -containing peptides were specifically detected on subsequent comprehensive liquid chromatography (LC)/MS analysis, and many O-glycosylation sites, including unreported ones, were identified. The position of glycosylated hydroxylysine, which is determined by our unambiguous and simple method, could provide insight into the physiological role of the modifications.
Figures

Similar articles
-
Site-specific quantitative analysis of overglycosylation of collagen in osteogenesis imperfecta using hydrazide chemistry and SILAC.J Proteome Res. 2013 May 3;12(5):2225-32. doi: 10.1021/pr400079d. Epub 2013 Apr 22. J Proteome Res. 2013. PMID: 23581850
-
Unusual fragmentation pathways in collagen glycopeptides.J Am Soc Mass Spectrom. 2013 Jul;24(7):1072-81. doi: 10.1007/s13361-013-0624-y. Epub 2013 Apr 30. J Am Soc Mass Spectrom. 2013. PMID: 23633013 Free PMC article.
-
LC-MS/MS identification of the O-glycosylation and hydroxylation of amino acid residues of collagen α-1 (II) chain from bovine cartilage.J Proteome Res. 2013 Aug 2;12(8):3599-609. doi: 10.1021/pr400101t. Epub 2013 Jul 23. J Proteome Res. 2013. PMID: 23879958 Free PMC article.
-
Collagen hydroxylysine glycosylation: non-conventional substrates for atypical glycosyltransferase enzymes.Biochem Soc Trans. 2021 Apr 30;49(2):855-866. doi: 10.1042/BST20200767. Biochem Soc Trans. 2021. PMID: 33704379 Review.
-
Advances in LC-MS/MS-based glycoproteomics: getting closer to system-wide site-specific mapping of the N- and O-glycoproteome.Biochim Biophys Acta. 2014 Sep;1844(9):1437-52. doi: 10.1016/j.bbapap.2014.05.002. Epub 2014 May 12. Biochim Biophys Acta. 2014. PMID: 24830338 Review.
Cited by
-
The Transcription Factor HAND1 Is Involved in Cortical Bone Mass through the Regulation of Collagen Expression.Int J Mol Sci. 2020 Nov 16;21(22):8638. doi: 10.3390/ijms21228638. Int J Mol Sci. 2020. PMID: 33207791 Free PMC article.
-
Type I and type V procollagen triple helix uses different subsets of the molecular ensemble for lysine posttranslational modifications in the rER.J Biol Chem. 2021 Jan-Jun;296:100453. doi: 10.1016/j.jbc.2021.100453. Epub 2021 Feb 23. J Biol Chem. 2021. PMID: 33631195 Free PMC article.
-
A Pragmatic Guide to Enrichment Strategies for Mass Spectrometry-Based Glycoproteomics.Mol Cell Proteomics. 2021;20:100029. doi: 10.1074/mcp.R120.002277. Epub 2020 Dec 20. Mol Cell Proteomics. 2021. PMID: 33583771 Free PMC article. Review.
-
Posttranslational modifications in type I collagen from different tissues extracted from wild type and prolyl 3-hydroxylase 1 null mice.J Biol Chem. 2013 Aug 23;288(34):24742-52. doi: 10.1074/jbc.M113.464156. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861401 Free PMC article.
-
Preservation of collagen in the soft tissues of frozen mammoths.PLoS One. 2021 Oct 29;16(10):e0258699. doi: 10.1371/journal.pone.0258699. eCollection 2021. PLoS One. 2021. PMID: 34714842 Free PMC article.
References
-
- Spiro R. G. (1967) The structure of the disaccharide unit of the renal glomerular basement membrane. J. Biol. Chem. 242, 4813–4823 - PubMed
-
- Wang C., Luosujärvi H., Heikkinen J., Risteli M., Uitto L., Myllylä R. (2002) The third activity for lysyl hydroxylase 3: galactosylation of hydroxylysyl residues in collagens in vitro. Matrix Biol 21, 559–566 - PubMed
-
- Kivirikko K. I., Myllylä R. (1979) Collagen glycosyltransferases. Int Rev Connect Tissue Res 8, 23–72 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

