Role of Mycobacterium tuberculosis pknD in the pathogenesis of central nervous system tuberculosis
- PMID: 22243650
- PMCID: PMC3322341
- DOI: 10.1186/1471-2180-12-7
Role of Mycobacterium tuberculosis pknD in the pathogenesis of central nervous system tuberculosis
Abstract
Background: Central nervous system disease is the most serious form of tuberculosis, and is associated with high mortality and severe neurological sequelae. Though recent clinical reports suggest an association of distinct Mycobacterium tuberculosis strains with central nervous system disease, the microbial virulence factors required have not been described previously.
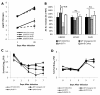
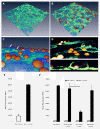
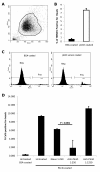
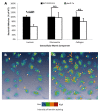
Results: We screened 398 unique M. tuberculosis mutants in guinea pigs to identify genes required for central nervous system tuberculosis. We found M. tuberculosis pknD (Rv0931c) to be required for central nervous system disease. These findings were central nervous system tissue-specific and were not observed in lung tissues. We demonstrated that pknD is required for invasion of brain endothelia (primary components of the blood-brain barrier protecting the central nervous system), but not macrophages, lung epithelia, or other endothelia. M. tuberculosis pknD encodes a "eukaryotic-like" serine-threonine protein kinase, with a predicted intracellular kinase and an extracellular (sensor) domain. Using confocal microscopy and flow cytometry we demonstrated that the M. tuberculosis PknD sensor is sufficient to trigger invasion of brain endothelia, a process which was neutralized by specific antiserum.
Conclusions: Our findings demonstrate a novel in vivo role for M. tuberculosis pknD and represent an important mechanism for bacterial invasion and virulence in central nervous system tuberculosis, a devastating and understudied disease primarily affecting young children.
Figures
Similar articles
-
Vaccination with recombinant Mycobacterium tuberculosis PknD attenuates bacterial dissemination to the brain in guinea pigs.PLoS One. 2013 Jun 11;8(6):e66310. doi: 10.1371/journal.pone.0066310. Print 2013. PLoS One. 2013. PMID: 23776655 Free PMC article.
-
Mycobacterium tuberculosis invasion and traversal across an in vitro human blood-brain barrier as a pathogenic mechanism for central nervous system tuberculosis.J Infect Dis. 2006 May 1;193(9):1287-95. doi: 10.1086/502631. Epub 2006 Mar 28. J Infect Dis. 2006. PMID: 16586367
-
Mycobacterium tuberculosis transporter MmpL7 is a potential substrate for kinase PknD.Biochem Biophys Res Commun. 2006 Sep 15;348(1):6-12. doi: 10.1016/j.bbrc.2006.06.164. Epub 2006 Jul 10. Biochem Biophys Res Commun. 2006. PMID: 16879801
-
Pathogenesis of central nervous system tuberculosis.Curr Mol Med. 2009 Mar;9(2):94-9. doi: 10.2174/156652409787581655. Curr Mol Med. 2009. PMID: 19275620 Free PMC article. Review.
-
Central nervous system tuberculosis: pathogenesis and clinical aspects.Clin Microbiol Rev. 2008 Apr;21(2):243-61, table of contents. doi: 10.1128/CMR.00042-07. Clin Microbiol Rev. 2008. PMID: 18400795 Free PMC article. Review.
Cited by
-
Genome sequence of the Drosophila melanogaster male-killing Spiroplasma strain MSRO endosymbiont.mBio. 2015 Mar 31;6(2):e02437-14. doi: 10.1128/mBio.02437-14. mBio. 2015. PMID: 25827421 Free PMC article.
-
Regulation of Ergothioneine Biosynthesis and Its Effect on Mycobacterium tuberculosis Growth and Infectivity.J Biol Chem. 2015 Sep 18;290(38):23064-76. doi: 10.1074/jbc.M115.648642. Epub 2015 Jul 30. J Biol Chem. 2015. PMID: 26229105 Free PMC article.
-
Tuberculous Meningitis in Children: Reducing the Burden of Death and Disability.Pathogens. 2021 Dec 30;11(1):38. doi: 10.3390/pathogens11010038. Pathogens. 2021. PMID: 35055986 Free PMC article. Review.
-
Pediatric tuberculosis in young children in India: a prospective study.Biomed Res Int. 2013;2013:783698. doi: 10.1155/2013/783698. Epub 2013 Dec 10. Biomed Res Int. 2013. PMID: 24386640 Free PMC article. Clinical Trial.
-
Large-scale genomic analysis shows association between homoplastic genetic variation in Mycobacterium tuberculosis genes and meningeal or pulmonary tuberculosis.BMC Genomics. 2018 Feb 5;19(1):122. doi: 10.1186/s12864-018-4498-z. BMC Genomics. 2018. PMID: 29402222 Free PMC article.
References
-
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, Zeller K, Andrews J, Friedland G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368(9547):1575–1580. doi: 10.1016/S0140-6736(06)69573-1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

