Lymphoid tissue damage in HIV-1 infection depletes naïve T cells and limits T cell reconstitution after antiretroviral therapy
- PMID: 22241988
- PMCID: PMC3252371
- DOI: 10.1371/journal.ppat.1002437
Lymphoid tissue damage in HIV-1 infection depletes naïve T cells and limits T cell reconstitution after antiretroviral therapy
Abstract
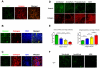
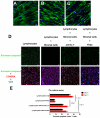
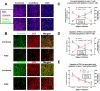
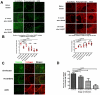
Highly active antiretroviral therapy (HAART) can suppress HIV-1 replication and normalize the chronic immune activation associated with infection, but restoration of naïve CD4+ T cell populations is slow and usually incomplete for reasons that have yet to be determined. We tested the hypothesis that damage to the lymphoid tissue (LT) fibroblastic reticular cell (FRC) network contributes to naïve T cell loss in HIV-1 infection by restricting access to critical factors required for T cell survival. We show that collagen deposition and progressive loss of the FRC network in LTs prior to treatment restrict both access to and a major source of the survival factor interleukin-7 (IL-7). As a consequence, apoptosis within naïve T cell populations increases significantly, resulting in progressive depletion of both naïve CD4+ and CD8+ T cell populations. We further show that the extent of loss of the FRC network and collagen deposition predict the extent of restoration of the naïve T cell population after 6 month of HAART, and that restoration of FRC networks correlates with the stage of disease at which the therapy is initiated. Because restoration of the FRC network and reconstitution of naïve T cell populations are only optimal when therapy is initiated in the early/acute stage of infection, our findings strongly suggest that HAART should be initiated as soon as possible. Moreover, our findings also point to the potential use of adjunctive anti-fibrotic therapies to avert or moderate the pathological consequences of LT fibrosis, thereby improving immune reconstitution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection.Clin Vaccine Immunol. 2006 May;13(5):556-60. doi: 10.1128/CVI.13.5.556-560.2006. Clin Vaccine Immunol. 2006. PMID: 16682476 Free PMC article.
-
Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution.Blood. 2012 Aug 30;120(9):1856-67. doi: 10.1182/blood-2012-03-418624. Epub 2012 May 21. Blood. 2012. PMID: 22613799 Free PMC article.
-
Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy.J Virol. 2003 Nov;77(21):11708-17. doi: 10.1128/jvi.77.21.11708-11717.2003. J Virol. 2003. PMID: 14557656 Free PMC article.
-
Lymphoid tissue structure and HIV-1 infection: life or death for T cells.Trends Immunol. 2012 Jun;33(6):306-14. doi: 10.1016/j.it.2012.04.002. Epub 2012 May 19. Trends Immunol. 2012. PMID: 22613276 Review.
-
Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues.Immunol Rev. 2013 Jul;254(1):65-77. doi: 10.1111/imr.12070. Immunol Rev. 2013. PMID: 23772615 Free PMC article. Review.
Cited by
-
Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo.PLoS Pathog. 2013 Feb;9(2):e1003148. doi: 10.1371/journal.ppat.1003148. Epub 2013 Feb 7. PLoS Pathog. 2013. PMID: 23408885 Free PMC article.
-
Neutralization activity in chronic HIV infection is characterized by a distinct programming of follicular helper CD4 T cells.bioRxiv [Preprint]. 2024 Aug 3:2024.07.31.605954. doi: 10.1101/2024.07.31.605954. bioRxiv. 2024. PMID: 39131331 Free PMC article. Preprint.
-
Lymphoid Tissue Mesenchymal Stromal Cells in Development and Tissue Remodeling.Stem Cells Int. 2016;2016:8419104. doi: 10.1155/2016/8419104. Epub 2016 Apr 13. Stem Cells Int. 2016. PMID: 27190524 Free PMC article. Review.
-
Examining Relationships between Metabolism and Persistent Inflammation in HIV Patients on Antiretroviral Therapy.Mediators Inflamm. 2018 Sep 27;2018:6238978. doi: 10.1155/2018/6238978. eCollection 2018. Mediators Inflamm. 2018. PMID: 30363715 Free PMC article. Review.
-
Visualizing Viral Infection In Vivo by Multi-Photon Intravital Microscopy.Viruses. 2018 Jun 20;10(6):337. doi: 10.3390/v10060337. Viruses. 2018. PMID: 29925766 Free PMC article. Review.
References
-
- Haase AT. Population biology of HIV-1 infection: viral and CD4+ T cell demographics and dynamics in lymphatic tissues. Annu Rev Immunol. 1999;17:625–656. - PubMed
-
- Andersson J, Fehniger TE, Patterson BK, Pottage J, Agnoli M, et al. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS. 1998;12:F123–129. - PubMed
-
- Lewden C, Salmon D, Morlat P, Bevilacqua S, Jougla E, et al. Causes of death among human immunodeficiency virus (HIV)-infected adults in the era of potent antiretroviral therapy: emerging role of hepatitis and cancers, persistent role of AIDS. Int J Epidemiol. 2005;34:121–130. - PubMed
-
- Barbaro G, Barbarini G. HIV infection and cancer in the era of highly active antiretroviral therapy (Review). Oncol Rep. 2007;17:1121–1126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

