Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress
- PMID: 22240589
- PMCID: PMC3336077
- DOI: 10.4161/auto.8.2.18600
Autophagy-dependent senescence in response to DNA damage and chronic apoptotic stress
Abstract
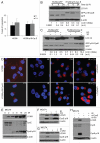
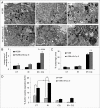
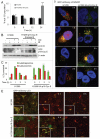
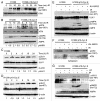
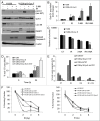
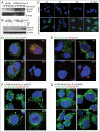
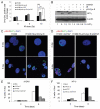
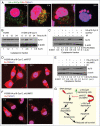
Autophagy regulates cell survival and cell death upon various cellular stresses, yet the molecular signaling events involved are not well defined. Here, we established the function of a proteolytic Cyclin E fragment (p18-CycE) in DNA damage-induced autophagy, apoptosis, and senescence. p18-CycE was identified in hematopoietic cells undergoing DNA damage-induced apoptosis. In epithelial cells exposed to DNA damage, chronic but not transient expression of p18-CycE leads to higher turnover of LC3 I/II and increased emergence of autophagosomes and autolysosomes. Levels of p18-CycE, which was generated by proteolytic cleavage of endogenous Cyclin E, were greatly increased by chloroquine and correlated with LC 3II conversion. Preventing p18-CycE genesis blocked conversion of LC3 I to LC3 II. Upon DNA damage, cytoplasmic ataxia-telangiectasia-mutated (ATM) was phosphorylated in p18-CycE-expressing cells resulting in sustained activation of the adenosine-mono-phosphate-dependent kinase (AMPK). These lead to sustained activation of mammalian autophagy-initiating kinase ULK1, which was abrogated upon inhibiting ATM and AMPK phosphorylation. Moreover, p18-CycE was degraded via autophagy followed by induction of senescence. Both autophagy and senescence were prevented by inhibiting autophagy, which leads to increased apoptosis in p18-CycE-expressing cells by stabilizing p18-CycE expression. Senescence was further associated with cytoplasmic co-localization and degradation of p18-CycE and Ku70. In brief, chronic p18-CycE expression-induced autophagy leads to clearance of p18-CycE following DNA damage and induction of senescence. Autophagy inhibition stabilized the cytoplasmic p18-CycE-Ku70 complex leading to apoptosis. Thus, our findings define how chronic apoptotic stress and DNA damage initiate autophagy and regulate cell survival through senescence and/or apoptosis.
Figures
Similar articles
-
Interaction of a cyclin E fragment with Ku70 regulates Bax-mediated apoptosis.Mol Cell Biol. 2007 May;27(9):3511-20. doi: 10.1128/MCB.01448-06. Epub 2007 Feb 26. Mol Cell Biol. 2007. PMID: 17325036 Free PMC article.
-
A C-terminal fragment of Cyclin E, generated by caspase-mediated cleavage, is degraded in the absence of a recognizable phosphodegron.J Biol Chem. 2008 Nov 7;283(45):30796-803. doi: 10.1074/jbc.M804642200. Epub 2008 Sep 10. J Biol Chem. 2008. PMID: 18784078 Free PMC article.
-
A jekyll and hyde role of cyclin E in the genotoxic stress response: switching from cell cycle control to apoptosis regulation.Cell Cycle. 2007 Jun 15;6(12):1437-42. doi: 10.4161/cc.6.12.4432. Epub 2007 May 10. Cell Cycle. 2007. PMID: 17581275 Free PMC article. Review.
-
Single-cell analysis challenges the connection between autophagy and senescence induced by DNA damage.Autophagy. 2015;11(7):1099-113. doi: 10.1080/15548627.2015.1009795. Autophagy. 2015. PMID: 25701485 Free PMC article.
-
Ku, Artemis, and ataxia-telangiectasia-mutated: signalling networks in DNA damage.Int J Biochem Cell Biol. 2008;40(4):598-603. doi: 10.1016/j.biocel.2007.12.007. Epub 2007 Dec 24. Int J Biochem Cell Biol. 2008. PMID: 18243767 Review.
Cited by
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
-
Autophagy through 4EBP1 and AMPK regulates oxidative stress-induced premature senescence in auditory cells.Oncotarget. 2015 Feb 28;6(6):3644-55. doi: 10.18632/oncotarget.2874. Oncotarget. 2015. PMID: 25682865 Free PMC article.
-
The DNA-damage response and nuclear events as regulators of nonapoptotic forms of cell death.Oncogene. 2020 Jan;39(1):1-16. doi: 10.1038/s41388-019-0980-6. Epub 2019 Aug 28. Oncogene. 2020. PMID: 31462710 Review.
-
Emerging Autophagy Functions Shape the Tumor Microenvironment and Play a Role in Cancer Progression - Implications for Cancer Therapy.Front Oncol. 2020 Nov 25;10:606436. doi: 10.3389/fonc.2020.606436. eCollection 2020. Front Oncol. 2020. PMID: 33324568 Free PMC article. Review.
-
CD47 deficiency confers cell and tissue radioprotection by activation of autophagy.Autophagy. 2012 Nov;8(11):1628-42. doi: 10.4161/auto.21562. Epub 2012 Aug 9. Autophagy. 2012. PMID: 22874555 Free PMC article.
References
-
- Chen Q, Gong B, Almasan A. Distinct stages of cytochrome c release from mitochondria: evidence for a feedback amplification loop linking caspase activation to mitochondrial dysfunction in genotoxic stress induced apoptosis. Cell Death Differ. 2000;7:227–233. doi: 10.1038/sj.cdd.4400629. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
