Alpha interferon and HIV infection cause activation of human T cells in NSG-BLT mice
- PMID: 22238321
- PMCID: PMC3302309
- DOI: 10.1128/JVI.06676-11
Alpha interferon and HIV infection cause activation of human T cells in NSG-BLT mice
Abstract
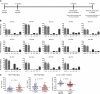
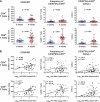
The development of small animal models for the study of HIV transmission is important for evaluation of HIV prophylaxis and disease pathogenesis. In humanized bone marrow-liver-thymus (BLT) mice, hematopoiesis is reconstituted by implantation of human fetal liver and thymus tissue (Thy/Liv) plus intravenous injection of autologous liver-derived hematopoietic stem progenitor cells (HSPC). This results in reconstitution of human leukocytes in the mouse peripheral blood, lymphoid organs, and mucosal sites. NOD-scid interleukin-2 receptor-negative (IL-2Rγ(-/-)) (NSG)-BLT mice were inoculated intravaginally with HIV and were monitored for plasma viremia by a branched DNA assay 4 weeks later. T-cell activation was determined by expression of CD38 and HLA-DR on human CD4(+) and CD8(+) T cells in mouse peripheral blood at the time of inoculation and 4 weeks later. Additional BLT mice were treated with human alpha interferon 2b (IFN-α2b) (intron A) and assessed for T-cell activation. Productive HIV infection in BLT mice was associated with T-cell activation (increases in CD38 mean fluorescence intensity and both the frequency and absolute number of CD38(+) HLA-DR(+) T cells) that correlated strongly with plasma viral load and was most pronounced in the CD8(+) T-cell compartment. This T-cell activation phenotype was recapitulated in NSG-BLT mice treated with intron A. HIV susceptibility correlated with the number of HSPC injected, yet a number of mice receiving the Thy/Liv implant alone, with no HSPC injection, were also susceptible to intravaginal HIV. These results are consistent with studies linking T-cell activation to progressive disease in humans and lend support for the use of NSG-BLT mice in studies of HIV pathogenesis.
Figures




Similar articles
-
Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rγ(-/-) (NSG) BLT mice.Virology. 2011 Aug 15;417(1):154-60. doi: 10.1016/j.virol.2011.05.013. Virology. 2011. PMID: 21684569 Free PMC article.
-
HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model.Retrovirology. 2013 Oct 24;10:121. doi: 10.1186/1742-4690-10-121. Retrovirology. 2013. PMID: 24156277 Free PMC article.
-
Immunization of BLT Humanized Mice Redirects T Cell Responses to Gag and Reduces Acute HIV-1 Viremia.J Virol. 2019 Sep 30;93(20):e00814-19. doi: 10.1128/JVI.00814-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375576 Free PMC article.
-
Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection.J Reprod Immunol. 2011 Mar;88(2):195-203. doi: 10.1016/j.jri.2010.11.005. Epub 2011 Jan 21. J Reprod Immunol. 2011. PMID: 21256601 Free PMC article. Review.
-
Current humanized mouse models for studying human immunology and HIV-1 immuno-pathogenesis.Sci China Life Sci. 2010 Feb;53(2):195-203. doi: 10.1007/s11427-010-0059-7. Epub 2010 Mar 7. Sci China Life Sci. 2010. PMID: 20596827 Free PMC article. Review.
Cited by
-
New generation humanized mice for virus research: comparative aspects and future prospects.Virology. 2013 Jan 5;435(1):14-28. doi: 10.1016/j.virol.2012.10.007. Virology. 2013. PMID: 23217612 Free PMC article. Review.
-
Crimean-Congo Hemorrhagic Fever in Humanized Mice Reveals Glial Cells as Primary Targets of Neurological Infection.J Infect Dis. 2017 Dec 12;216(11):1386-1397. doi: 10.1093/infdis/jix215. J Infect Dis. 2017. PMID: 28482001 Free PMC article.
-
Humanized Mice for the Evaluation of Novel HIV-1 Therapies.Front Immunol. 2021 Apr 1;12:636775. doi: 10.3389/fimmu.2021.636775. eCollection 2021. Front Immunol. 2021. PMID: 33868262 Free PMC article. Review.
-
In vivo analysis of highly conserved Nef activities in HIV-1 replication and pathogenesis.Retrovirology. 2013 Oct 30;10:125. doi: 10.1186/1742-4690-10-125. Retrovirology. 2013. PMID: 24172637 Free PMC article.
-
Moving beyond the mousetrap: current and emerging humanized mouse and rat models for investigating prevention and cure strategies against HIV infection and associated pathologies.Retrovirology. 2020 Apr 10;17(1):8. doi: 10.1186/s12977-020-00515-3. Retrovirology. 2020. PMID: 32276640 Free PMC article. Review.
References
-
- Brenchley JM, et al. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371 - PubMed
-
- Choudhary SK, et al. 2009. Suppression of human immunodeficiency virus type 1 (HIV-1) viremia with reverse transcriptase and integrase inhibitors, CD4+ T-cell recovery, and viral rebound upon interruption of therapy in a new model for HIV treatment in the humanized Rag2−/− γc−/− mouse. J. Virol. 83:8254–8258 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

