Generation of autologous tumor-specific T cells for adoptive transfer based on vaccination, in vitro restimulation and CD3/CD28 dynabead-induced T cell expansion
- PMID: 22237888
- PMCID: PMC11029656
- DOI: 10.1007/s00262-011-1199-8
Generation of autologous tumor-specific T cells for adoptive transfer based on vaccination, in vitro restimulation and CD3/CD28 dynabead-induced T cell expansion
Abstract
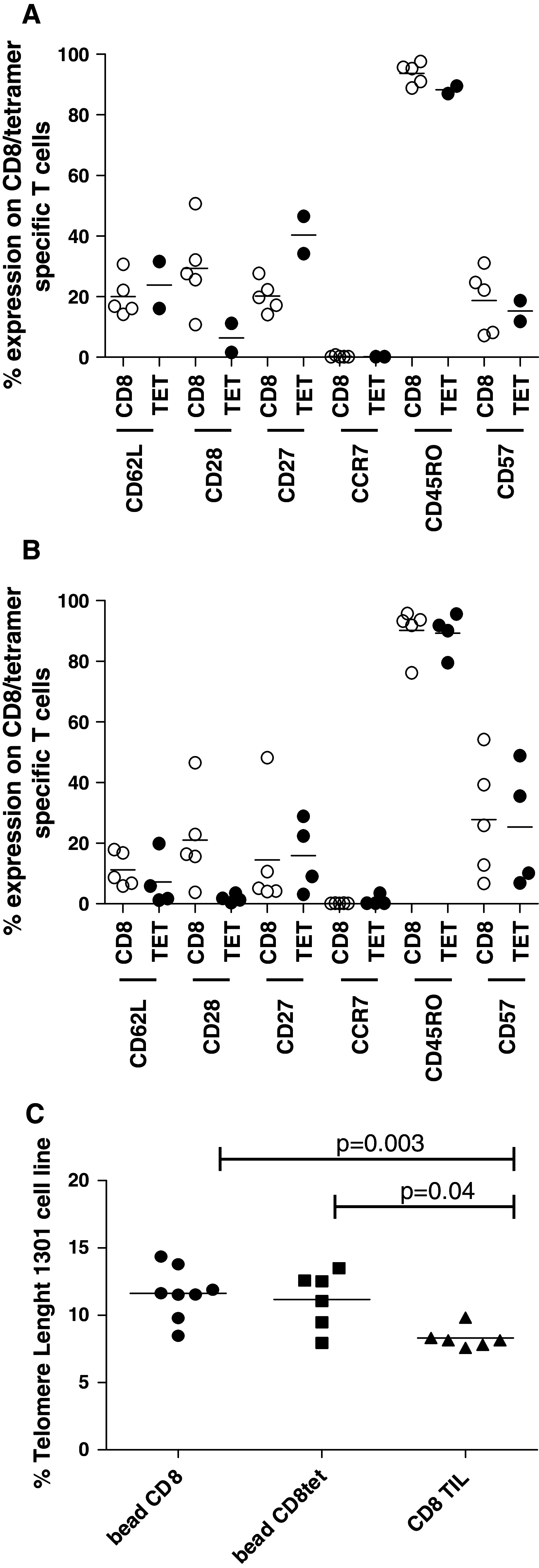
Adoptive cell transfer (ACT) of in vitro expanded autologous tumor-infiltrating lymphocytes (TIL) has been shown to exert therapeutic efficacy in melanoma patients. We aimed to develop an ACT protocol based on tumor-specific T cells isolated from peripheral blood and in vitro expanded by Dynabeads® ClinExVivo™CD3/CD28. We show here that the addition of an in vitro restimulation step with relevant peptides prior to bead expansion dramatically increased the proportion of tumor-specific T cells in PBMC-cultures. Importantly, peptide-pulsed dendritic cells (DCs) as well as allogeneic tumor lysate-pulsed DCs from the DC vaccine preparation could be used with comparable efficiency to peptides for in vitro restimulation, to increase the tumor-specific T-cell response. Furthermore, we tested the use of different ratios and different types of Dynabeads® CD3/CD28 and CD3/CD28/CD137 T-cell expander, for optimized expansion of tumor-specific T cells. A ratio of 1:3 of Dynabeads® CD3/CD28 T-cell expander to T cells resulted in the maximum number of tumor-specific T cells. The addition of CD137 did not improve functionality or fold expansion. Both T-cell expansion systems could generate tumor-specific T cells that were both cytotoxic and effective cytokine producers upon antigen recognition. Dynabeads®-expanded T-cell cultures shows phenotypical characteristics of memory T cells with potential to migrate and expand in vivo. In addition, they possess longer telomeres compared to TIL cultures. Taken together, we demonstrate that in vitro restimulation of tumor-specific T cells prior to bead expansion is necessary to achieve high numbers of tumor-specific T cells. This is effective and easily applicable in combination with DC vaccination, by use of vaccine-generated DCs, either pulsed with peptide or tumor-lysate.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Optimization of human T-cell expansion ex vivo using magnetic beads conjugated with anti-CD3 and Anti-CD28 antibodies.J Immunother. 2004 Sep-Oct;27(5):405-18. doi: 10.1097/00002371-200409000-00010. J Immunother. 2004. PMID: 15314550
-
MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro.J Immunol. 2010 Jan 1;184(1):452-65. doi: 10.4049/jimmunol.0901101. Epub 2009 Nov 30. J Immunol. 2010. PMID: 19949105
-
Ex vivo expansion of dendritic-cell-activated antigen-specific CD4+ T cells with anti-CD3/CD28, interleukin-7, and interleukin-15: potential for adoptive T cell immunotherapy.Clin Immunol. 2006 Apr;119(1):21-31. doi: 10.1016/j.clim.2005.11.003. Epub 2006 Jan 9. Clin Immunol. 2006. PMID: 16406844
-
In vitro stimulation and expansion of human tumour-reactive CD8+ cytotoxic T lymphocytes by anti-CD3/CD28/CD137 magnetic beads.Scand J Immunol. 2011 Aug;74(2):155-64. doi: 10.1111/j.1365-3083.2011.02564.x. Scand J Immunol. 2011. PMID: 21517928
-
Dendritic cell-based combined immunotherapy with autologous tumor-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives.Hum Cell. 2003 Dec;16(4):175-82. doi: 10.1111/j.1749-0774.2003.tb00151.x. Hum Cell. 2003. PMID: 15147037 Review.
Cited by
-
Similarity and difference in tumor-infiltrating lymphocytes in original tumor tissues and those of in vitro expanded populations in head and neck cancer.Oncotarget. 2017 Dec 19;9(3):3805-3814. doi: 10.18632/oncotarget.23454. eCollection 2018 Jan 9. Oncotarget. 2017. PMID: 29423084 Free PMC article.
-
Trial Watch: Adoptive cell transfer for anticancer immunotherapy.Oncoimmunology. 2013 May 1;2(5):e24238. doi: 10.4161/onci.24238. Oncoimmunology. 2013. PMID: 23762803 Free PMC article.
-
Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients.J Transl Med. 2012 Aug 21;10:169. doi: 10.1186/1479-5876-10-169. J Transl Med. 2012. PMID: 22909342 Free PMC article.
-
CXCR1 as a novel target for directing reactive T cells toward melanoma: implications for adoptive cell transfer immunotherapy.Cancer Immunol Immunother. 2012 Oct;61(10):1833-47. doi: 10.1007/s00262-012-1245-1. Epub 2012 Mar 24. Cancer Immunol Immunother. 2012. PMID: 22441657 Free PMC article.
-
Inhibition of Calcium Signaling Prevents Exhaustion and Enhances Anti-Leukemia Efficacy of CAR-T Cells via SOCE-Calcineurin-NFAT and Glycolysis Pathways.Adv Sci (Weinh). 2022 Mar;9(9):e2103508. doi: 10.1002/advs.202103508. Epub 2022 Jan 14. Adv Sci (Weinh). 2022. PMID: 35032108 Free PMC article.
References
-
- Donia M, Junker N, Ellebaek E, Andersen MH, Straten PT, Svane IM (2011) Characterization and comparison of “standard” and “young” tumor infiltrating lymphocytes for adoptive cell therapy at a Danish Translational Research Institution. Scand J Immunol [Epub ahead of print] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

