"A novel in vivo model for the study of human breast cancer metastasis using primary breast tumor-initiating cells from patient biopsies"
- PMID: 22233382
- PMCID: PMC3277457
- DOI: 10.1186/1471-2407-12-10
"A novel in vivo model for the study of human breast cancer metastasis using primary breast tumor-initiating cells from patient biopsies"
Abstract
Background: The study of breast cancer metastasis depends on the use of established breast cancer cell lines that do not accurately represent the heterogeneity and complexity of human breast tumors. A tumor model was developed using primary breast tumor-initiating cells isolated from patient core biopsies that would more accurately reflect human breast cancer metastasis.
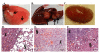
Methods: Tumorspheres were isolated under serum-free culture conditions from core biopsies collected from five patients with clinical diagnosis of invasive ductal carcinoma (IDC). Isolated tumorspheres were transplanted into the mammary fat pad of NUDE mice to establish tumorigenicity in vivo. Tumors and metastatic lesions were analyzed by hematoxylin and eosin (H+E) staining and immunohistochemistry (IHC).
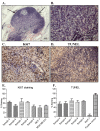
Results: Tumorspheres were successfully isolated from all patient core biopsies, independent of the estrogen receptor α (ERα)/progesterone receptor (PR)/Her2/neu status or tumor grade. Each tumorsphere was estimated to contain 50-100 cells. Transplantation of 50 tumorspheres (1-5 × 103 cells) in combination with Matrigel into the mammary fat pad of NUDE mice resulted in small, palpable tumors that were sustained up to 12 months post-injection. Tumors were serially transplanted three times by re-isolation of tumorspheres from the tumors and injection into the mammary fat pad of NUDE mice. At 3 months post-injection, micrometastases to the lung, liver, kidneys, brain and femur were detected by measuring content of human chromosome 17. Visible macrometastases were detected in the lung, liver and kidneys by 6 months post-injection. Primary tumors variably expressed cytokeratins, Her2/neu, cytoplasmic E-cadherin, nuclear β catenin and fibronectin but were negative for ERα and vimentin. In lung and liver metastases, variable redistribution of E-cadherin and β catenin to the membrane of tumor cells was observed. ERα was re-expressed in lung metastatic cells in two of five samples.
Conclusions: Tumorspheres isolated under defined culture conditions from patient core biopsies were tumorigenic when transplanted into the mammary fat pad of NUDE mice, and metastasized to multiple mouse organs. Micrometastases in mouse organs demonstrated a dormancy period prior to outgrowth of macrometastases. The development of macrometastases with organ-specific phenotypic distinctions provides a superior model for the investigation of organ-specific effects on metastatic cancer cell survival and growth.
Figures



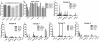

Similar articles
-
Disseminated breast cancer cells acquire a highly malignant and aggressive metastatic phenotype during metastatic latency in the bone.PLoS One. 2012;7(11):e47587. doi: 10.1371/journal.pone.0047587. Epub 2012 Nov 15. PLoS One. 2012. PMID: 23173031 Free PMC article.
-
Metastasis tumor antigen family proteins during breast cancer progression and metastasis in a reliable mouse model for human breast cancer.Clin Cancer Res. 2006 Mar 1;12(5):1479-86. doi: 10.1158/1078-0432.CCR-05-1519. Clin Cancer Res. 2006. PMID: 16533771
-
A mouse model for luminal epithelial like ER positive subtype of human breast cancer.BMC Cancer. 2007 Sep 20;7:180. doi: 10.1186/1471-2407-7-180. BMC Cancer. 2007. PMID: 17880731 Free PMC article.
-
Nontransgenic models of breast cancer.Breast Cancer Res. 2000;2(5):331-4. doi: 10.1186/bcr77. Epub 2000 Aug 4. Breast Cancer Res. 2000. PMID: 11250725 Free PMC article. Review.
-
Breast cancer brain metastasis: from etiology to state-of-the-art modeling.J Biol Eng. 2023 Jun 29;17(1):41. doi: 10.1186/s13036-023-00352-w. J Biol Eng. 2023. PMID: 37386445 Free PMC article. Review.
Cited by
-
Peptidomimetic Src/pretubulin inhibitor KX-01 alone and in combination with paclitaxel suppresses growth, metastasis in human ER/PR/HER2-negative tumor xenografts.Mol Cancer Ther. 2012 Sep;11(9):1936-47. doi: 10.1158/1535-7163.MCT-12-0146. Epub 2012 Jul 10. Mol Cancer Ther. 2012. PMID: 22784709 Free PMC article.
-
Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts.PLoS One. 2014 Feb 28;9(2):e89595. doi: 10.1371/journal.pone.0089595. eCollection 2014. PLoS One. 2014. PMID: 24586900 Free PMC article.
-
Breast cancer in sub-Saharan Africa: The current state and uncertain future.Exp Biol Med (Maywood). 2021 Jun;246(12):1377-1387. doi: 10.1177/15353702211006047. Epub 2021 Apr 29. Exp Biol Med (Maywood). 2021. PMID: 33926257 Free PMC article. Review.
-
Suppression of triple-negative breast cancer metastasis by pan-DAC inhibitor panobinostat via inhibition of ZEB family of EMT master regulators.Breast Cancer Res Treat. 2014 Jun;145(3):593-604. doi: 10.1007/s10549-014-2979-6. Epub 2014 May 9. Breast Cancer Res Treat. 2014. PMID: 24810497 Free PMC article.
-
Molecular biomarkers of cancer stem/progenitor cells associated with progression, metastases, and treatment resistance of aggressive cancers.Cancer Epidemiol Biomarkers Prev. 2014 Feb;23(2):234-54. doi: 10.1158/1055-9965.EPI-13-0785. Epub 2013 Nov 22. Cancer Epidemiol Biomarkers Prev. 2014. PMID: 24273063 Free PMC article. Review.
References
-
- Allan AL, Vantyghem SA, Tuck AB, Chambers AF. Tumor dormancy and cancer stem cells: implications for the biology and treatment of breast cancer metastasis. Breast Dis. 2006;26:87–98. - PubMed
-
- Giovanella BC, Vardeman DM, Williams LJ, Taylor DJ, De Ipolyi PD, Greeff PJ. et al.Heterotransplantation of human breast carcinomas in nude mice. Correlation between successful heterotransplants, poor prognosis and amplification of the HER-2/neu oncogene. Int J Cancer. 1991;47(1):66–71. doi: 10.1002/ijc.2910470113. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

