Pancreatic polypeptide administration enhances insulin sensitivity and reduces the insulin requirement of patients on insulin pump therapy
- PMID: 22226275
- PMCID: PMC3262724
- DOI: 10.1177/193229681100500629
Pancreatic polypeptide administration enhances insulin sensitivity and reduces the insulin requirement of patients on insulin pump therapy
Abstract
Introduction: The effects of pancreatic polypeptide (PP) infusion were examined in patients on insulin pump therapy to determine whether PP administration can reduce insulin requirements in patients with type 1 diabetes mellitus (T1DM) or type 3c diabetes mellitus (T3cDM; pancreatogenic).
Methods: Ten subjects with long-standing T1DM (n = 7) or T3cDM (n = 3) on insulin pump treatment received a 72 h subcutaneous infusion of 2 pmol/kg/min bovine PP or saline by portable infusion pump in a single-blinded, randomized, crossover design.
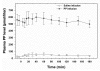
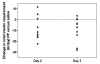
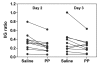
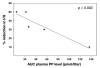
Results: Pancreatic polypeptide infusion raised plasma PP levels to 450-700 pmol/liter. Daily insulin infusion requirements (I) fell from 48 ± 6.9 to 40 ± 7.5 U on day 2 (p < .05) and from 46 ± 7.7 to 37 ± 6.6 U on day 3 (p < .05) of PP infusion compared with saline. Corrected for average blood glucose concentration (G), I/G fell in 10/10 subjects during the second 24 h period and in 7/10 subjects during the third 24 h period; sensitivity to insulin, calculated as 1/(I/G), increased 45% ± 12% on day 2 (p < .01) and 34% ± 14% on day 3 (p < .05) of PP infusion. Pancreatic polypeptide responses to a test meal were compared with the change in insulin infusion requirements in 5 subjects; the reduction in insulin requirements seen during PP infusion correlated with the degree of baseline PP deficiency (p < .002).
Conclusions: A concurrent subcutaneous infusion of PP enhances insulin sensitivity and reduces insulin requirements in patients with long-standing T1DM and T3cDM on insulin pump therapy. The benefit of PP infusion correlated with the degree of PP deficiency.
© 2011 Diabetes Technology Society.
Figures
Similar articles
-
Reversal of abnormal glucose production after pancreatic resection by pancreatic polypeptide administration in man.Surgery. 1988 Aug;104(2):119-29. Surgery. 1988. PMID: 3041640
-
Pancreatic polypeptide administration improves abnormal glucose metabolism in patients with chronic pancreatitis.J Clin Endocrinol Metab. 1996 Oct;81(10):3566-72. doi: 10.1210/jcem.81.10.8855802. J Clin Endocrinol Metab. 1996. PMID: 8855802
-
Pancreatic polypeptide administration reduces insulin requirements of artificial pancreas in pancreatectomized dogs.Artif Organs. 2005 Jan;29(1):83-7. doi: 10.1111/j.1525-1594.2004.29008.x. Artif Organs. 2005. PMID: 15644089
-
Pancreatogenic diabetes: special considerations for management.Pancreatology. 2011;11(3):279-94. doi: 10.1159/000329188. Epub 2011 Jul 9. Pancreatology. 2011. PMID: 21757968 Review.
-
Approach to the adult hospitalized patient on an insulin pump.J Hosp Med. 2013 Dec;8(12):721-7. doi: 10.1002/jhm.2109. Epub 2013 Nov 13. J Hosp Med. 2013. PMID: 24227761 Review.
Cited by
-
Diabetes of the Exocrine Pancreas Related to Hereditary Pancreatitis, an Update.Curr Diab Rep. 2020 Mar 28;20(6):16. doi: 10.1007/s11892-020-01299-8. Curr Diab Rep. 2020. PMID: 32221727 Review.
-
To Evaluate the Effect of Edrophonium on Blood Glucose Levels in Euglycemic Albino Rats Through OGTT.J Clin Diagn Res. 2015 Jan;9(1):FF04-7. doi: 10.7860/JCDR/2015/11073.5452. Epub 2015 Jan 1. J Clin Diagn Res. 2015. PMID: 25738004 Free PMC article.
-
Signaling Molecules Regulating Pancreatic Endocrine Development from Pluripotent Stem Cell Differentiation.Int J Mol Sci. 2020 Aug 15;21(16):5867. doi: 10.3390/ijms21165867. Int J Mol Sci. 2020. PMID: 32824212 Free PMC article. Review.
-
Therapeutic peptides: current applications and future directions.Signal Transduct Target Ther. 2022 Feb 14;7(1):48. doi: 10.1038/s41392-022-00904-4. Signal Transduct Target Ther. 2022. PMID: 35165272 Free PMC article. Review.
-
A Mediterranean-like fat blend protects against the development of severe colitis in the mucin-2 deficient murine model.Gut Microbes. 2022 Jan-Dec;14(1):2055441. doi: 10.1080/19490976.2022.2055441. Gut Microbes. 2022. PMID: 35471119 Free PMC article.
References
-
- Taylor IL. Pancreatic polypeptide family: pancreatic polypeptide, neuropeptide Y and peptide YY. 2nd ed. Bethesda: American Physiological Society; 1989.
-
- Kono T, Wang XP, Fisher WE, Andersen DK, Brunicardi FC. Pancreatic polypeptide. In: Martini L, editor. Encyclopedia of endocrine diseases. Vol. 3. San Diego: Elsevier; 2004. pp. 488–496.
-
- Schwartz TW, Rehfeld JF, Stadil F, Larson LI, Chance RE, Moon N. Pancreatic-polypeptide response to food in duodenal-ulcer patients before and after vagotomy. Lancet. 1976;1(7969):1102–1105. - PubMed
-
- Orci L, Malaisse-Lagae F, Baetens D, Perrelet A. Pancreatic-poly-peptide-rich regions in human pancreas. Lancet. 1978;2(8101):1200–1201. - PubMed
-
- Hazelwood RL. The pancreatic polypeptide (PP-fold) family: gastro-intestinal, vascular, and feeding behavioral implications. Proc Soc Exp Biol Med. 1993;202(1):44–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

