Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours
- PMID: 22223088
- PMCID: PMC3273358
- DOI: 10.1038/bjc.2011.555
Phase I study to assess the safety and tolerability of olaparib in combination with bevacizumab in patients with advanced solid tumours
Abstract
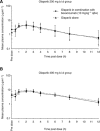
Background: Olaparib (AZD2281) is a potent oral poly(ADP-ribose) polymerase inhibitor with anti-tumour activity and acceptable toxicity as monotherapy in patients with BRCA-deficient cancers. The vascular endothelial growth factor receptor inhibitor bevacizumab has been incorporated into standard of care with chemotherapy in various tumours. This phase I study established the safety, tolerability and clinical pharmacokinetics of olaparib alone and in combination with bevacizumab.
Methods: Patients with advanced solid tumours received increasing doses of continuous oral olaparib (100, 200 and 400 mg b.i.d. capsule formulation) in combination with bevacizumab (10 mg kg(-1) intravenous q2w).
Results: In all, 12 patients enrolled and received treatment. The most common adverse events (AEs) related to olaparib were grade 1/2 nausea and fatigue. No haematological parameters were reported as AEs. No serious AEs related to olaparib or dose-limiting toxicities (DLTs) were reported. Three patients discontinued due to AEs, two patients discontinued both olaparib and bevacizumab and one patient discontinued olaparib. Five patients received combination treatment for over 6 months. There was no evidence that bevacizumab affected olaparib.
Conclusion: The combination of olaparib 400 mg b.i.d. with bevacizumab 10 mg kg(-1) q2w was generally well tolerated with no DLTs. This combination could be considered for future clinical investigation.
Figures
Similar articles
-
A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer.Eur J Cancer. 2013 Sep;49(14):2972-8. doi: 10.1016/j.ejca.2013.05.020. Epub 2013 Jun 27. Eur J Cancer. 2013. PMID: 23810467 Free PMC article. Clinical Trial.
-
Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study.Invest New Drugs. 2012 Aug;30(4):1493-500. doi: 10.1007/s10637-011-9682-9. Epub 2011 May 18. Invest New Drugs. 2012. PMID: 21590367 Clinical Trial.
-
Safety and Clinical Activity of the Programmed Death-Ligand 1 Inhibitor Durvalumab in Combination With Poly (ADP-Ribose) Polymerase Inhibitor Olaparib or Vascular Endothelial Growth Factor Receptor 1-3 Inhibitor Cediranib in Women's Cancers: A Dose-Escalation, Phase I Study.J Clin Oncol. 2017 Jul 1;35(19):2193-2202. doi: 10.1200/JCO.2016.72.1340. Epub 2017 May 4. J Clin Oncol. 2017. PMID: 28471727 Free PMC article. Clinical Trial.
-
Cediranib, a pan-VEGFR inhibitor, and olaparib, a PARP inhibitor, in combination therapy for high grade serous ovarian cancer.Expert Opin Investig Drugs. 2016;25(5):597-611. doi: 10.1517/13543784.2016.1156857. Epub 2016 Mar 16. Expert Opin Investig Drugs. 2016. PMID: 26899229 Review.
-
Patient Counseling and Management of Symptoms During Olaparib Therapy for Recurrent Ovarian Cancer.Oncologist. 2016 Aug;21(8):954-63. doi: 10.1634/theoncologist.2015-0268. Epub 2016 Jun 2. Oncologist. 2016. PMID: 27256873 Free PMC article. Review.
Cited by
-
Targeting hypoxia in solid and haematological malignancies.J Exp Clin Cancer Res. 2022 Nov 2;41(1):318. doi: 10.1186/s13046-022-02522-y. J Exp Clin Cancer Res. 2022. PMID: 36320041 Free PMC article. Review.
-
Dual inhibition of VEGF and PARP suppresses KRAS-mutant colorectal cancer.Neoplasia. 2020 Sep;22(9):365-375. doi: 10.1016/j.neo.2020.06.001. Epub 2020 Jul 3. Neoplasia. 2020. PMID: 32629177 Free PMC article.
-
Progress in the treatment of ovarian cancer-lessons from homologous recombination deficiency-the first 10 years.Ann Oncol. 2016 Apr;27 Suppl 1(Suppl 1):i1-i3. doi: 10.1093/annonc/mdw082. Ann Oncol. 2016. PMID: 27141062 Free PMC article. Review.
-
Anti-angiogenic agents in ovarian cancer: past, present, and future.Ann Oncol. 2016 Apr;27 Suppl 1(Suppl 1):i33-i39. doi: 10.1093/annonc/mdw093. Ann Oncol. 2016. PMID: 27141068 Free PMC article. Review.
-
A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer.Eur J Cancer. 2013 Sep;49(14):2972-8. doi: 10.1016/j.ejca.2013.05.020. Epub 2013 Jun 27. Eur J Cancer. 2013. PMID: 23810467 Free PMC article. Clinical Trial.
References
-
- Agency EM (2011) Avastin: European Public Assessment Report EMEA/H/C/000582-II/0040
-
- Aghajanian C, Finkler NJ, Rutherford T, Teneriello MG, Yi J, Parmar H, Nycum LR, Sovak MA (2011) OCEANS: a randomized, double-blinded, placebo-controlled phase III trial of chemotherapy with or without bevacizumab (BEV) in patients with platinum-sensitive recurrent epithelial ovarian (EOC), primary peritoneal (PPC), or fallopian tube cancer (FTC). J Clin Oncol 29(suppl): abst LBA5007 - PMC - PubMed
-
- Ashworth A (2008) A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol 26: 3785–3790 - PubMed
-
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, Scott C, Weitzel JN, Oaknin A, Loman N, Lu K, Schmutzler RK, Matulonis U, Wickens M, Tutt A (2010) Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376: 245–251 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources