Immunomodulatory functions of type I interferons
- PMID: 22222875
- PMCID: PMC3727154
- DOI: 10.1038/nri3133
Immunomodulatory functions of type I interferons
Abstract
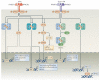
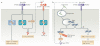
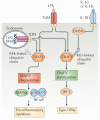
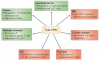
Interferon-α (IFNα) and IFNβ, collectively known as type I IFNs, are the major effector cytokines of the host immune response against viral infections. However, the production of type I IFNs is also induced in response to bacterial ligands of innate immune receptors and/or bacterial infections, indicating a broader physiological role for these cytokines in host defence and homeostasis than was originally assumed. The main focus of this Review is the underappreciated immunomodulatory functions of type I IFNs in health and disease. We discuss their function in the regulation of innate and adaptive immune responses, the response to bacterial ligands, inflammasome activation, intestinal homeostasis and inflammatory and autoimmune diseases.
Figures
Similar articles
-
Innate Antiviral Defenses Independent of Inducible IFNα/β Production.Trends Immunol. 2016 Sep;37(9):588-596. doi: 10.1016/j.it.2016.06.003. Epub 2016 Jun 23. Trends Immunol. 2016. PMID: 27345728 Review.
-
Shared and Unique Features of Human Interferon-Beta and Interferon-Alpha Subtypes.Front Immunol. 2021 Jan 19;11:605673. doi: 10.3389/fimmu.2020.605673. eCollection 2020. Front Immunol. 2021. PMID: 33542718 Free PMC article. Review.
-
The yin and yang of viruses and interferons.Trends Immunol. 2012 Apr;33(4):190-7. doi: 10.1016/j.it.2012.01.004. Epub 2012 Feb 7. Trends Immunol. 2012. PMID: 22321608 Free PMC article. Review.
-
Context Is Key: Delineating the Unique Functions of IFNα and IFNβ in Disease.Front Immunol. 2020 Dec 21;11:606874. doi: 10.3389/fimmu.2020.606874. eCollection 2020. Front Immunol. 2020. PMID: 33408718 Free PMC article. Review.
-
Crosstalk between type I and II interferons in regulation of myeloid cell responses during bacterial infection.Curr Opin Immunol. 2018 Oct;54:35-41. doi: 10.1016/j.coi.2018.05.014. Epub 2018 Jun 7. Curr Opin Immunol. 2018. PMID: 29886270 Free PMC article. Review.
Cited by
-
Repurposing Drugs, Ongoing Vaccine, and New Therapeutic Development Initiatives Against COVID-19.Front Pharmacol. 2020 Aug 19;11:1258. doi: 10.3389/fphar.2020.01258. eCollection 2020. Front Pharmacol. 2020. PMID: 32973505 Free PMC article. Review.
-
Transcriptional Repression of IFN Regulatory Factor 7 by MYC Is Critical for Type I IFN Production in Human Plasmacytoid Dendritic Cells.J Immunol. 2016 Oct 15;197(8):3348-3359. doi: 10.4049/jimmunol.1502385. Epub 2016 Sep 14. J Immunol. 2016. PMID: 27630164 Free PMC article.
-
T-Cell Exhaustion in HIV-1/Hepatitis C Virus Coinfection Is Reduced After Successful Treatment of Chronic Hepatitis C.Open Forum Infect Dis. 2023 Oct 21;10(11):ofad514. doi: 10.1093/ofid/ofad514. eCollection 2023 Nov. Open Forum Infect Dis. 2023. PMID: 37953817 Free PMC article.
-
Fueling autoimmunity: type I interferon in autoimmune diseases.Expert Rev Clin Immunol. 2013 Mar;9(3):201-10. doi: 10.1586/eci.12.106. Expert Rev Clin Immunol. 2013. PMID: 23445195 Free PMC article. Review.
-
Pretreatment of UC-MSCs with IFN-α2 improves treatment of liver fibrosis by recruiting neutrophils.J Transl Med. 2023 Nov 18;21(1):832. doi: 10.1186/s12967-023-04732-0. J Transl Med. 2023. PMID: 37980535 Free PMC article.
References
-
- Salaman RN. Protective inoculation against a plant virus. Nature. 1933;131:468.
-
- White PB. Lysogenic strains of V. cholerae and the influence of lysozyme on cholera phage activity. J. Pathol. Bacteriol. 1937;44:276–278.
-
- Hoskins M. A protective action of neurotropic against viscerotropic yellow fever virus in Macacus rhesus. Am. J. Trop. Med. 1935;15:675–680.
-
- Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 1957;147:258–267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

