Oligomerization of ZFYVE27 (Protrudin) is necessary to promote neurite extension
- PMID: 22216323
- PMCID: PMC3247280
- DOI: 10.1371/journal.pone.0029584
Oligomerization of ZFYVE27 (Protrudin) is necessary to promote neurite extension
Abstract
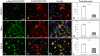
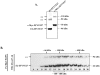
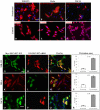
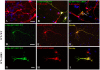
ZFYVE27 (Protrudin) was originally identified as an interacting partner of spastin, which is most frequently mutated in hereditary spastic paraplegia. ZFYVE27 is a novel member of FYVE family, which is implicated in the formation of neurite extensions by promoting directional membrane trafficking in neurons. Now, through a yeast two-hybrid screen, we have identified that ZFYVE27 interacts with itself and the core interaction region resides within the third hydrophobic region (HR3) of the protein. We confirmed the ZFYVE27's self-interaction in the mammalian cells by co-immunoprecipitation and co-localization studies. To decipher the oligomeric nature of ZFYVE27, we performed sucrose gradient centrifugation and showed that ZFYVE27 oligomerizes into dimer/tetramer forms. Sub-cellular fractionation and Triton X-114 membrane phase separation analysis indicated that ZFYVE27 is a peripheral membrane protein. Furthermore, ZFYVE27 also binds to phosphatidylinositol 3-phosphate lipid moiety. Interestingly, cells expressing ZFYVE27(ΔHR3) failed to produce protrusions instead caused swelling of cell soma. When ZFYVE27(ΔHR3) was co-expressed with wild-type ZFYVE27 (ZFYVE27(WT)), it exerted a dominant negative effect on ZFYVE27(WT) as the cells co-expressing both proteins were also unable to induce protrusions and showed cytoplasmic swelling. Altogether, it is evident that a functionally active form of oligomer is crucial for ZFYVE27 ability to promote neurite extensions.
© 2011 Pantakani et al.
Conflict of interest statement
Figures




Similar articles
-
Phosphoinositides differentially regulate protrudin localization through the FYVE domain.J Biol Chem. 2012 Nov 30;287(49):41268-76. doi: 10.1074/jbc.M112.419127. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043110 Free PMC article.
-
ZFYVE27 (SPG33), a novel spastin-binding protein, is mutated in hereditary spastic paraplegia.Am J Hum Genet. 2006 Aug;79(2):351-7. doi: 10.1086/504927. Epub 2006 Jun 1. Am J Hum Genet. 2006. PMID: 16826525 Free PMC article.
-
Protrudin regulates endoplasmic reticulum morphology and function associated with the pathogenesis of hereditary spastic paraplegia.J Biol Chem. 2014 May 9;289(19):12946-61. doi: 10.1074/jbc.M113.528687. Epub 2014 Mar 25. J Biol Chem. 2014. PMID: 24668814 Free PMC article.
-
Roles of protrudin at interorganelle membrane contact sites.Proc Jpn Acad Ser B Phys Biol Sci. 2019;95(7):312-320. doi: 10.2183/pjab.95.023. Proc Jpn Acad Ser B Phys Biol Sci. 2019. PMID: 31406056 Free PMC article. Review.
-
[Mechanism for neurite formation via recycling of membrane].Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2202-6. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038609 Review. Japanese. No abstract available.
Cited by
-
Phosphoinositides differentially regulate protrudin localization through the FYVE domain.J Biol Chem. 2012 Nov 30;287(49):41268-76. doi: 10.1074/jbc.M112.419127. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043110 Free PMC article.
-
Fatty acid synthase cooperates with protrudin to facilitate membrane outgrowth of cellular protrusions.Sci Rep. 2017 Apr 21;7:46569. doi: 10.1038/srep46569. Sci Rep. 2017. PMID: 28429738 Free PMC article.
-
Protrudin regulates FAK activation, endothelial cell migration and angiogenesis.Cell Mol Life Sci. 2022 Apr 4;79(4):220. doi: 10.1007/s00018-022-04251-z. Cell Mol Life Sci. 2022. PMID: 35368213 Free PMC article.
-
Protrudin binds atlastins and endoplasmic reticulum-shaping proteins and regulates network formation.Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):14954-9. doi: 10.1073/pnas.1307391110. Epub 2013 Aug 22. Proc Natl Acad Sci U S A. 2013. PMID: 23969831 Free PMC article.
-
Repeated ER-endosome contacts promote endosome translocation and neurite outgrowth.Nature. 2015 Apr 9;520(7546):234-8. doi: 10.1038/nature14359. Nature. 2015. PMID: 25855459
References
-
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. - PubMed
-
- Horton AC, Ehlers MD. Neuronal polarity and trafficking. Neuron. 2003;40:277–295. - PubMed
-
- Wang L, Brown A. Rapid movement of microtubules in axons. Curr Biol. 2002;12:1496–1501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

