Epstein-Barr virus evades CD4+ T cell responses in lytic cycle through BZLF1-mediated downregulation of CD74 and the cooperation of vBcl-2
- PMID: 22216005
- PMCID: PMC3245307
- DOI: 10.1371/journal.ppat.1002455
Epstein-Barr virus evades CD4+ T cell responses in lytic cycle through BZLF1-mediated downregulation of CD74 and the cooperation of vBcl-2
Abstract
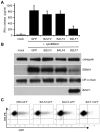
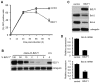
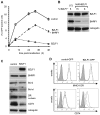
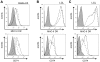
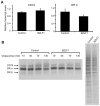
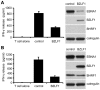
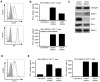
Evasion of immune T cell responses is crucial for viruses to establish persistence in the infected host. Immune evasion mechanisms of Epstein-Barr virus (EBV) in the context of MHC-I antigen presentation have been well studied. In contrast, viral interference with MHC-II antigen presentation is less well understood, not only for EBV but also for other persistent viruses. Here we show that the EBV encoded BZLF1 can interfere with recognition by immune CD4+ effector T cells. This impaired T cell recognition occurred in the absence of a reduction in the expression of surface MHC-II, but correlated with a marked downregulation of surface CD74 on the target cells. Furthermore, impaired CD4+ T cell recognition was also observed with target cells where CD74 expression was downregulated by shRNA-mediated inhibition. BZLF1 downregulated surface CD74 via a post-transcriptional mechanism distinct from its previously reported effect on the CIITA promoter. In addition to being a chaperone for MHC-II αβ dimers, CD74 also functions as a surface receptor for macrophage Migration Inhibitory Factor and enhances cell survival through transcriptional upregulation of Bcl-2 family members. The immune-evasion function of BZLF1 therefore comes at a cost of induced toxicity. However, during EBV lytic cycle induced by BZLF1 expression, this toxicity can be overcome by expression of the vBcl-2, BHRF1, at an early stage of lytic infection. We conclude that by inhibiting apoptosis, the vBcl-2 not only maintains cell viability to allow sufficient time for synthesis and accumulation of infectious virus progeny, but also enables BZLF1 to effect its immune evasion function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The Missing Link in Epstein-Barr Virus Immune Evasion: the BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II.J Virol. 2015 Oct 14;90(1):356-67. doi: 10.1128/JVI.02183-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468525 Free PMC article.
-
The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules.J Virol. 2002 Aug;76(16):8179-88. doi: 10.1128/jvi.76.16.8179-8188.2002. J Virol. 2002. PMID: 12134023 Free PMC article.
-
Epstein-Barr virus LMP2A suppresses MHC class II expression by regulating the B-cell transcription factors E47 and PU.1.Blood. 2015 Apr 2;125(14):2228-38. doi: 10.1182/blood-2014-08-594689. Epub 2015 Jan 28. Blood. 2015. PMID: 25631773
-
Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review.
-
Immunodominance of lytic cycle antigens in Epstein-Barr virus-specific CD4+ T cell preparations for therapy.PLoS One. 2007 Jul 4;2(7):e583. doi: 10.1371/journal.pone.0000583. PLoS One. 2007. PMID: 17611619 Free PMC article. Review.
Cited by
-
An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model.J Virol. 2012 Aug;86(15):7976-87. doi: 10.1128/JVI.00770-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623780 Free PMC article.
-
An updated view of the pathogenesis of steroid-sensitive nephrotic syndrome.Pediatr Nephrol. 2022 Sep;37(9):1957-1965. doi: 10.1007/s00467-021-05401-4. Epub 2022 Jan 10. Pediatr Nephrol. 2022. PMID: 35006356 Free PMC article. Review.
-
Identification of Epstein-Barr Virus Replication Proteins in Burkitt's Lymphoma Cells.Pathogens. 2015 Oct 29;4(4):739-51. doi: 10.3390/pathogens4040739. Pathogens. 2015. PMID: 26529022 Free PMC article.
-
S-Like-Phase Cyclin-Dependent Kinases Stabilize the Epstein-Barr Virus BDLF4 Protein To Temporally Control Late Gene Transcription.J Virol. 2019 Apr 3;93(8):e01707-18. doi: 10.1128/JVI.01707-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30700607 Free PMC article.
-
Immune escape of γ-herpesviruses from adaptive immunity.Rev Med Virol. 2014 Nov;24(6):365-78. doi: 10.1002/rmv.1791. Epub 2014 Apr 15. Rev Med Virol. 2014. PMID: 24733560 Free PMC article. Review.
References
-
- Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Walters Kluwer/Lippincott, Williams & Wilkins; 2007. pp. 2655–2700.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

