Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover
- PMID: 22214853
- PMCID: PMC3248306
- DOI: 10.1172/JCI60016
Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover
Abstract
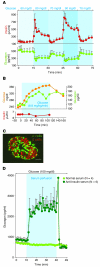
The hormone glucagon has long been dismissed as a minor contributor to metabolic disease. Here we propose that glucagon excess, rather than insulin deficiency, is the sine qua non of diabetes. We base this on the following evidence: (a) glucagon increases hepatic glucose and ketone production, catabolic features present in insulin deficiency; (b) hyperglucagonemia is present in every form of poorly controlled diabetes; (c) the glucagon suppressors leptin and somatostatin suppress all catabolic manifestations of diabetes during total insulin deficiency; (d) total β cell destruction in glucagon receptor-null mice does not cause diabetes; and (e) perfusion of normal pancreas with anti-insulin serum causes marked hyperglucagonemia. From this and other evidence, we conclude that glucose-responsive β cells normally regulate juxtaposed α cells and that without intraislet insulin, unregulated α cells hypersecrete glucagon, which directly causes the symptoms of diabetes. This indicates that glucagon suppression or inactivation may provide therapeutic advantages over insulin monotherapy.
Figures



Similar articles
-
Glucagon is the key factor in the development of diabetes.Diabetologia. 2016 Jul;59(7):1372-1375. doi: 10.1007/s00125-016-3965-9. Epub 2016 Apr 26. Diabetologia. 2016. PMID: 27115412 Review.
-
Role of hyperglucagonemia in catabolism associated with type 1 diabetes: effects on leucine metabolism and the resting metabolic rate.Diabetes. 1998 Nov;47(11):1748-56. doi: 10.2337/diabetes.47.11.1748. Diabetes. 1998. PMID: 9792544
-
Alterations in somatostatin and other islet cell functions in the spontaneously diabetic BB Wistar rat: biochemical and morphological characterization.Metabolism. 1983 Jul;32(7 Suppl 1):18-25. doi: 10.1016/s0026-0495(83)80006-7. Metabolism. 1983. PMID: 6135136
-
Insulin, glucagon, and somatostatin in normal physiology and diabetes mellitus.Diabetes. 1976 Dec;25(12):1091-9. doi: 10.2337/diab.25.12.1091. Diabetes. 1976. PMID: 992227
-
Glucagon and diabetes.Med Clin North Am. 1978 Jul;62(4):713-22. doi: 10.1016/s0025-7125(16)31767-9. Med Clin North Am. 1978. PMID: 355737 Review.
Cited by
-
d-Amino Acids and Classical Neurotransmitters in Healthy and Type 2 Diabetes-Affected Human Pancreatic Islets of Langerhans.Metabolites. 2022 Aug 27;12(9):799. doi: 10.3390/metabo12090799. Metabolites. 2022. PMID: 36144204 Free PMC article.
-
Exercise-Induced Improvements to Whole Body Glucose Metabolism in Type 2 Diabetes: The Essential Role of the Liver.Front Endocrinol (Lausanne). 2020 Aug 28;11:567. doi: 10.3389/fendo.2020.00567. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32982968 Free PMC article. Review.
-
Molecular basis for negative regulation of the glucagon receptor.Proc Natl Acad Sci U S A. 2012 Sep 4;109(36):14393-8. doi: 10.1073/pnas.1206734109. Epub 2012 Aug 20. Proc Natl Acad Sci U S A. 2012. PMID: 22908259 Free PMC article.
-
Small Mouse Islets Are Deficient in Glucagon-Producing Alpha Cells but Rich in Somatostatin-Secreting Delta Cells.J Diabetes Res. 2016;2016:4930741. doi: 10.1155/2016/4930741. Epub 2016 Jul 18. J Diabetes Res. 2016. PMID: 27504459 Free PMC article.
-
Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3.Endocrine. 2018 Dec;62(3):576-587. doi: 10.1007/s12020-018-1689-y. Epub 2018 Aug 16. Endocrine. 2018. PMID: 30117113
References
-
- Kimball CP, Murlin JR. Aqueous extracts of pancreas. III. Some precipitation reactions of insulin. J Biol Chem. 1923;58(1):337–346.
-
- Keller U, Chiasson JL, Liljenquist JE, Cherrington AD, Jennings AS, Crofford OS. The roles of insulin, glucagon, and free fatty acids in the regulation of ketogenesis in dogs. Diabetes. 1977;26(11):1040–1051. - PubMed
-
- Cherrington AD, Chiasson JL, Liljenquist JE, Lacy WW, Park CR. Control of hepatic glucose output by glucagon and insulin in the intact dog. Biochem Soc Symp. 1978;43(43):31–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

