Glutathione peroxidase 4 is required for maturation of photoreceptor cells
- PMID: 22207760
- PMCID: PMC3293550
- DOI: 10.1074/jbc.M111.335174
Glutathione peroxidase 4 is required for maturation of photoreceptor cells
Abstract
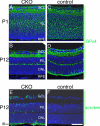
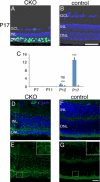
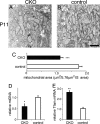
Oxidative stress is implicated in the pathologies of photoreceptor cells, and the protective role of antioxidant enzymes for photoreceptor cells have been well understood. However, their essentiality has remained unknown. In this study we generated photoreceptor-specific conditional knock-out (CKO) mice of glutathione peroxidase 4 (GPx4) and showed the critical role of GPx4 for photoreceptor cells. In the wild-type retina the dominant GPx4 expression was in the mitochondria, indicating the mitochondrial variant was the major GPx4 in the retina. In the GPx4-CKO mice, although photoreceptor cells developed and differentiated into rod and cone cells by P12, they rapidly underwent drastic degeneration and completely disappeared by P21. The photoreceptor cell death in the GPx4-CKO mice was associated with the nuclear translocation of apoptosis-inducing factor (AIF) and TUNEL-positive cells. Photoreceptor cells before undergoing apoptosis (P11) exhibited decreased mitochondrial biomass, decreased number of connecting cilia, as well as disorganized structure of outer segments. These findings indicate that GPx4 is a critical antioxidant enzyme for the maturation and survival of photoreceptor cells.
Figures





Similar articles
-
Mitochondrial glutathione peroxidase 4 is indispensable for photoreceptor development and survival in mice.J Biol Chem. 2022 Apr;298(4):101824. doi: 10.1016/j.jbc.2022.101824. Epub 2022 Mar 11. J Biol Chem. 2022. PMID: 35288190 Free PMC article.
-
Overexpression of SOD in retina: need for increase in H2O2-detoxifying enzyme in same cellular compartment.Free Radic Biol Med. 2011 Oct 1;51(7):1347-54. doi: 10.1016/j.freeradbiomed.2011.06.010. Epub 2011 Jul 5. Free Radic Biol Med. 2011. PMID: 21736939 Free PMC article.
-
Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis.J Biol Chem. 2004 Dec 31;279(53):55137-46. doi: 10.1074/jbc.M410387200. Epub 2004 Oct 20. J Biol Chem. 2004. PMID: 15496407
-
Function and regulation of GPX4 in the development and progression of fibrotic disease.J Cell Physiol. 2022 Jul;237(7):2808-2824. doi: 10.1002/jcp.30780. Epub 2022 May 23. J Cell Physiol. 2022. PMID: 35605092 Review.
-
Molecular biology of glutathione peroxidase 4: from genomic structure to developmental expression and neural function.Biol Chem. 2007 Oct;388(10):1007-17. doi: 10.1515/BC.2007.126. Biol Chem. 2007. PMID: 17937614 Review.
Cited by
-
Iron Accumulation and Lipid Peroxidation in the Aging Retina: Implication of Ferroptosis in Age-Related Macular Degeneration.Aging Dis. 2021 Apr 1;12(2):529-551. doi: 10.14336/AD.2020.0912. eCollection 2021 Apr. Aging Dis. 2021. PMID: 33815881 Free PMC article. Review.
-
Retinitis Pigmentosa: Progress in Molecular Pathology and Biotherapeutical Strategies.Int J Mol Sci. 2022 Apr 28;23(9):4883. doi: 10.3390/ijms23094883. Int J Mol Sci. 2022. PMID: 35563274 Free PMC article. Review.
-
Astragaloside A Protects Against Photoreceptor Degeneration in Part Through Suppressing Oxidative Stress and DNA Damage-Induced Necroptosis and Inflammation in the Retina.J Inflamm Res. 2022 May 20;15:2995-3020. doi: 10.2147/JIR.S362401. eCollection 2022. J Inflamm Res. 2022. PMID: 35645574 Free PMC article.
-
Iron contributes to photoreceptor degeneration and Müller glia proliferation in the zebrafish light-treated retina.Exp Eye Res. 2022 Mar;216:108947. doi: 10.1016/j.exer.2022.108947. Epub 2022 Jan 21. Exp Eye Res. 2022. PMID: 35074344 Free PMC article.
-
Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD).Biogerontology. 2013 Oct;14(5):461-82. doi: 10.1007/s10522-013-9463-2. Epub 2013 Sep 22. Biogerontology. 2013. PMID: 24057278 Free PMC article. Review.
References
-
- Tanito M., Masutani H., Nakamura H., Ohira A., Yodoi J. (2002) Cytoprotective effect of thioredoxin against retinal photic injury in mice. Invest. Ophthalmol. Vis. Sci. 43, 1162–1167 - PubMed
-
- Okoye G., Zimmer J., Sung J., Gehlbach P., Deering T., Nambu H., Hackett S., Melia M., Esumi N., Zack D. J., Campochiaro P. A. (2003) Increased expression of brain-derived neurotrophic factor preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J. Neurosci. 23, 4164–4172 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

