Targeting HIV-1 envelope glycoprotein trimers to B cells by using APRIL improves antibody responses
- PMID: 22205734
- PMCID: PMC3302291
- DOI: 10.1128/JVI.06259-11
Targeting HIV-1 envelope glycoprotein trimers to B cells by using APRIL improves antibody responses
Abstract
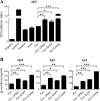
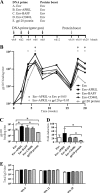
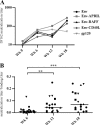
An HIV-1 vaccine remains elusive, in part because various factors limit the quantity and quality of the antibodies raised against the viral envelope glycoprotein complex (Env). We hypothesized that targeting Env vaccines directly to B cells, by fusing them to molecules that bind and activate these cells, would improve Env-specific antibody responses. Therefore, we fused trimeric Env gp140 to A PRoliferation-Inducing Ligand (APRIL), B-cell Activating Factor (BAFF), and CD40 Ligand (CD40L). The Env-APRIL, Env-BAFF, and Env-CD40L gp140 trimers all enhanced the expression of activation-induced cytidine deaminase (AID), the enzyme responsible for inducing somatic hypermutation, antibody affinity maturation, and antibody class switching. They also triggered IgM, IgG, and IgA secretion from human B cells in vitro. The Env-APRIL trimers induced higher anti-Env antibody responses in rabbits, including neutralizing antibodies against tier 1 viruses. The enhanced Env-specific responses were not associated with a general increase in total plasma antibody concentrations, indicating that the effect of APRIL was specific for Env. All the rabbit sera raised against gp140 trimers, irrespective of the presence of CD40L, BAFF, or APRIL, recognized trimeric Env efficiently, whereas sera raised against gp120 monomers did not. The levels of trimer-binding and virus-neutralizing antibodies were strongly correlated, suggesting that gp140 trimers are superior to gp120 monomers as immunogens. Targeting and activating B cells with a trimeric HIV-1 Env-APRIL fusion protein may therefore improve the induction of humoral immunity against HIV-1.
Figures
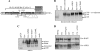


Similar articles
-
DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers.J Virol. 2015 Apr;89(8):4158-69. doi: 10.1128/JVI.02904-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631080 Free PMC article.
-
Enhanced immunogenicity of HIV-1 envelope gp140 proteins fused to APRIL.PLoS One. 2014 Sep 23;9(9):e107683. doi: 10.1371/journal.pone.0107683. eCollection 2014. PLoS One. 2014. PMID: 25247707 Free PMC article.
-
Trimeric HIV-1 gp140 fused with APRIL, BAFF, and CD40L on the mucosal gp140-specific antibody responses in mice.Vaccine. 2020 Feb 24;38(9):2149-2159. doi: 10.1016/j.vaccine.2020.01.050. Epub 2020 Jan 31. Vaccine. 2020. PMID: 32014267
-
Humoral immunity to HIV-1: neutralisation and antibody effector functions.Trends Microbiol. 2008 Dec;16(12):596-604. doi: 10.1016/j.tim.2008.08.008. Epub 2008 Oct 27. Trends Microbiol. 2008. PMID: 18964020 Review.
-
Targeting APRIL in the treatment of glomerular diseases.Kidney Int. 2024 Nov;106(5):806-818. doi: 10.1016/j.kint.2024.08.012. Epub 2024 Sep 7. Kidney Int. 2024. PMID: 39182759 Review.
Cited by
-
An HIV-1 envelope glycoprotein trimer with an embedded IL-21 domain activates human B cells.PLoS One. 2013 Jun 24;8(6):e67309. doi: 10.1371/journal.pone.0067309. Print 2013. PLoS One. 2013. PMID: 23826263 Free PMC article.
-
APRIL:TACI axis is dispensable for the immune response to rabies vaccination.Antiviral Res. 2017 Aug;144:130-137. doi: 10.1016/j.antiviral.2017.06.004. Epub 2017 Jun 12. Antiviral Res. 2017. PMID: 28619678 Free PMC article.
-
DNA vaccine molecular adjuvants SP-D-BAFF and SP-D-APRIL enhance anti-gp120 immune response and increase HIV-1 neutralizing antibody titers.J Virol. 2015 Apr;89(8):4158-69. doi: 10.1128/JVI.02904-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631080 Free PMC article.
-
Enhanced immunogenicity of HIV-1 envelope gp140 proteins fused to APRIL.PLoS One. 2014 Sep 23;9(9):e107683. doi: 10.1371/journal.pone.0107683. eCollection 2014. PLoS One. 2014. PMID: 25247707 Free PMC article.
-
Chimeric HIV-1 envelope glycoproteins with potent intrinsic granulocyte-macrophage colony-stimulating factor (GM-CSF) activity.PLoS One. 2013;8(4):e60126. doi: 10.1371/journal.pone.0060126. Epub 2013 Apr 2. PLoS One. 2013. PMID: 23565193 Free PMC article.
References
-
- Beddows S, et al. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329–340 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI82362/AI/NIAID NIH HHS/United States
- 280829/ERC_/European Research Council/International
- R56 AI058763/AI/NIAID NIH HHS/United States
- R01 AI058763/AI/NIAID NIH HHS/United States
- U01 AI095613/AI/NIAID NIH HHS/United States
- R37 AI036082/AI/NIAID NIH HHS/United States
- R37 AI36082/AI/NIAID NIH HHS/United States
- R33 AI084714/AI/NIAID NIH HHS/United States
- P01 AI061093/AI/NIAID NIH HHS/United States
- AI84714/AI/NIAID NIH HHS/United States
- R21 AI084714/AI/NIAID NIH HHS/United States
- AI58763/AI/NIAID NIH HHS/United States
- U19 AI096187/AI/NIAID NIH HHS/United States
- R01 AI057653/AI/NIAID NIH HHS/United States
- R01 AI074378/AI/NIAID NIH HHS/United States
- P01 AI082362/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

