Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils
- PMID: 22203955
- PMCID: PMC3258617
- DOI: 10.1073/pnas.1110996109
Gastrin-releasing peptide receptor (GRPR) mediates chemotaxis in neutrophils
Abstract
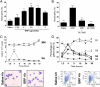
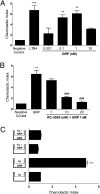
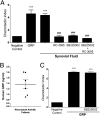
Neutrophil migration to inflamed sites is crucial for both the initiation of inflammation and resolution of infection, yet these cells are involved in perpetuation of different chronic inflammatory diseases. Gastrin-releasing peptide (GRP) is a neuropeptide that acts through G protein coupled receptors (GPCRs) involved in signal transmission in both central and peripheral nervous systems. Its receptor, gastrin-releasing peptide receptor (GRPR), is expressed by various cell types, and it is overexpressed in cancer cells. RC-3095 is a selective GRPR antagonist, recently found to have antiinflammatory properties in arthritis and sepsis models. Here we demonstrate that i.p. injection of GRP attracts neutrophils in 4 h, and attraction is blocked by RC-3095. Macrophage depletion or neutralization of TNF abrogates GRP-induced neutrophil recruitment to the peritoneum. In vitro, GRP-induced neutrophil migration was dependent on PLC-β2, PI3K, ERK, p38 and independent of Gαi protein, and neutrophil migration toward synovial fluid of arthritis patients was inhibited by treatment with RC-3095. We propose that GRPR is an alternative chemotactic receptor that may play a role in the pathogenesis of inflammatory disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
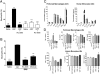

Similar articles
-
Gastrin-releasing peptide as a molecular target for inflammatory diseases: an update.Inflamm Allergy Drug Targets. 2013 Jun;12(3):172-7. doi: 10.2174/1871528111312030003. Inflamm Allergy Drug Targets. 2013. PMID: 23621446 Review.
-
Gastrin-Releasing Peptide Is Involved in the Establishment of Allergic Rhinitis in Mice.Laryngoscope. 2018 Nov;128(11):E377-E384. doi: 10.1002/lary.27394. Epub 2018 Aug 27. Laryngoscope. 2018. PMID: 30151920
-
Gastrin-releasing peptide receptor antagonism induces protection from lethal sepsis: involvement of toll-like receptor 4 signaling.Mol Med. 2012 Oct 24;18(1):1209-19. doi: 10.2119/molmed.2012.00083. Mol Med. 2012. PMID: 22735756 Free PMC article. Clinical Trial.
-
GRPR antagonist protects from drug-induced liver injury by impairing neutrophil chemotaxis and motility.Eur J Immunol. 2017 Apr;47(4):646-657. doi: 10.1002/eji.201646394. Eur J Immunol. 2017. PMID: 28294319
-
Novel insight on GRP/GRPR axis in diseases.Biomed Pharmacother. 2023 May;161:114497. doi: 10.1016/j.biopha.2023.114497. Epub 2023 Mar 16. Biomed Pharmacother. 2023. PMID: 36933382 Review.
Cited by
-
n-butanol extract from Folium isatidis inhibits the lipopolysaccharide-induced downregulation of CXCR1 and CXCR2 on human neutrophils.Mol Med Rep. 2018 Jan;17(1):179-185. doi: 10.3892/mmr.2017.7870. Epub 2017 Oct 25. Mol Med Rep. 2018. PMID: 29115434 Free PMC article.
-
α-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation.Proc Natl Acad Sci U S A. 2015 Apr 14;112(15):E1926-35. doi: 10.1073/pnas.1417883112. Epub 2015 Mar 30. Proc Natl Acad Sci U S A. 2015. PMID: 25825709 Free PMC article.
-
Innate Receptors Expression by Lung Nociceptors: Impact on COVID-19 and Aging.Front Immunol. 2021 Dec 16;12:785355. doi: 10.3389/fimmu.2021.785355. eCollection 2021. Front Immunol. 2021. PMID: 34975876 Free PMC article. Review.
-
Phosphorylation of iRhom2 Controls Stimulated Proteolytic Shedding by the Metalloprotease ADAM17/TACE.Cell Rep. 2017 Oct 17;21(3):745-757. doi: 10.1016/j.celrep.2017.09.074. Cell Rep. 2017. PMID: 29045841 Free PMC article.
-
Immune Actions on the Peripheral Nervous System in Pain.Int J Mol Sci. 2021 Feb 1;22(3):1448. doi: 10.3390/ijms22031448. Int J Mol Sci. 2021. PMID: 33535595 Free PMC article. Review.
References
-
- Burbach JP. Neuropeptides from concept to online database www.neuropeptides.nl. Eur J Pharmacol. 2010;626(1):27–48. - PubMed
-
- Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. - PubMed
-
- Hernanz A, Tato E, De la Fuente M, de Miguel E, Arnalich F. Differential effects of gastrin-releasing peptide, neuropeptide Y, somatostatin and vasoactive intestinal peptide on interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha production by whole blood cells from healthy young and old subjects. J Neuroimmunol. 1996;71:25–30. - PubMed
-
- McDonald TJ, et al. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979;90:227–233. - PubMed
-
- Xiao D, Wang J, Hampton LL, Weber HC. The human gastrin-releasing peptide receptor gene structure, its tissue expression and promoter. Gene. 2001;264:95–103. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

