CD90 is identified as a candidate marker for cancer stem cells in primary high-grade gliomas using tissue microarrays
- PMID: 22203689
- PMCID: PMC3433900
- DOI: 10.1074/mcp.M111.010744
CD90 is identified as a candidate marker for cancer stem cells in primary high-grade gliomas using tissue microarrays
Abstract
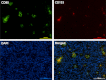
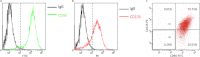
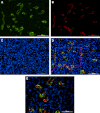
Although CD90 has been identified as a marker for various kinds of stem cells including liver cancer stem cells (CSCs) that are responsible for tumorigenesis, the potential role of CD90 as a marker for CSCs in gliomas has not been characterized. To address the issue, we investigated the expression of CD90 in tissue microarrays containing 15 glioblastoma multiformes (GBMs), 19 WHO grade III astrocytomas, 13 WHO grade II astrocytomas, 3 WHO grade I astrocytomas and 8 normal brain tissues. Immunohistochemical analysis showed that CD90 was expressed at a medium to high level in all tested high-grade gliomas (grade III and GBM) whereas it was barely detectable in low-grade gliomas (grade I and grade II) and normal brains. Double immunofluorescence staining for CD90 and CD133 in GBM tissues revealed that CD133(+) CSCs are a subpopulation of CD90(+) cells in GBMs in vivo. Flow cytometry analysis of the expression of CD90 and CD133 in GBM-derived stem-like neurospheres further confirmed the conclusion in vitro. The expression levels of both CD90 and CD133 were reduced along with the loss of stem cells after differentiation. Furthermore, the limiting dilution assay demonstrated that the sphere formation ability was comparable between the CD90(+)/CD133(+) and the CD90(+)/CD133(-) populations of GBM neurospheres, which is much higher than that of the CD90(-)/CD133(-) population. We also performed double staining for CD90 and a vascular endothelial cell marker CD31 in tissue microarrays which revealed that the CD90(+) cells were clustered around the tumor vasculatures in high-grade glioma tissues. These findings suggest that CD90 is not only a potential prognostic marker for high-grade gliomas but also a marker for CSCs within gliomas, and it resides within endothelial niche and may also play a critical role in the generation of tumor vasculatures via differentiation into endothelial cells.
Figures



Similar articles
-
Tenascin-C: a novel candidate marker for cancer stem cells in glioblastoma identified by tissue microarrays.J Proteome Res. 2015 Feb 6;14(2):814-22. doi: 10.1021/pr5008653. Epub 2014 Dec 12. J Proteome Res. 2015. PMID: 25469866 Free PMC article.
-
The prognostic value of clinical factors and cancer stem cell-related markers in gliomas.Dan Med J. 2014 Oct;61(10):B4944. Dan Med J. 2014. PMID: 25283629
-
[Correlative study of distribution of brain tumor stem cell with micro-vascular system].Zhonghua Yi Xue Za Zhi. 2010 Feb 2;90(5):305-9. Zhonghua Yi Xue Za Zhi. 2010. PMID: 20368050 Chinese.
-
CD133 as a marker for regulation and potential for targeted therapies in glioblastoma multiforme.Neurosurg Clin N Am. 2012 Jul;23(3):391-405. doi: 10.1016/j.nec.2012.04.011. Epub 2012 Jun 5. Neurosurg Clin N Am. 2012. PMID: 22748652 Review.
-
How powerful is CD133 as a cancer stem cell marker in brain tumors?Cancer Treat Rev. 2009 Aug;35(5):403-8. doi: 10.1016/j.ctrv.2009.03.002. Epub 2009 Apr 14. Cancer Treat Rev. 2009. PMID: 19369008 Review.
Cited by
-
Overexpression of Thy1 and ITGA6 is associated with invasion, metastasis and poor prognosis in human gallbladder carcinoma.Oncol Lett. 2016 Dec;12(6):5136-5144. doi: 10.3892/ol.2016.5341. Epub 2016 Nov 2. Oncol Lett. 2016. PMID: 28105220 Free PMC article.
-
Stemness-Related Markers in Cancer.Cancer Transl Med. 2017;3(3):87-95. doi: 10.4103/ctm.ctm_69_16. Epub 2017 Jun 8. Cancer Transl Med. 2017. PMID: 29276782 Free PMC article.
-
Current understanding of epigenetics mechanism as a novel target in reducing cancer stem cells resistance.Clin Epigenetics. 2021 May 29;13(1):120. doi: 10.1186/s13148-021-01107-4. Clin Epigenetics. 2021. PMID: 34051847 Free PMC article. Review.
-
Thy1 (CD90) expression is regulated by DNA methylation during adipogenesis.FASEB J. 2019 Mar;33(3):3353-3363. doi: 10.1096/fj.201801481R. Epub 2018 Oct 30. FASEB J. 2019. PMID: 30376360 Free PMC article.
-
Upregulation of miR-216a-5p by Lentinan Targeted Inhibition of JAK2/STAT3 Signaling Pathway to Reduce Lung Adenocarcinoma Cell Stemness, Promote Apoptosis, and Slow Down the Lung Adenocarcinoma Mechanisms.Front Oncol. 2021 Nov 25;11:778096. doi: 10.3389/fonc.2021.778096. eCollection 2021. Front Oncol. 2021. PMID: 34900727 Free PMC article.
References
-
- Ohgaki H., Dessen P., Jourde B., Horstmann S., Nishikawa T., Di Patre P. L., Burkhard C., Schüler D., Probst-Hensch N. M., Maiorka P. C., Baeza N., Pisani P., Yonekawa Y., Yasargil M. G., Lütolf U. M., Kleihues P. (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res. 64, 6892–6899 - PubMed
-
- Reardon D. A., Rich J. N., Friedman H. S., Bigner D. D. (2006) Recent advances in the treatment of malignant astrocytoma. J. Clin. Oncol. 24, 1253–1265 - PubMed
-
- Reya T., Morrison S. J., Clarke M. F., Weissman I. L. (2001) Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 - PubMed
-
- Visvader J. E., Lindeman G. J. (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer 8, 755–768 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

