Diethyldithiocarbamate induces apoptosis in HHV-8-infected primary effusion lymphoma cells via inhibition of the NF-κB pathway
- PMID: 22200846
- PMCID: PMC3584624
- DOI: 10.3892/ijo.2011.1313
Diethyldithiocarbamate induces apoptosis in HHV-8-infected primary effusion lymphoma cells via inhibition of the NF-κB pathway
Abstract
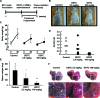
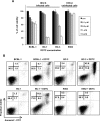
Primary effusion lymphoma (PEL) is a subtype of B-cell lymphoma caused by human herpes virus 8/Kaposi sarcoma-associated herpes virus (HHV-8/KSHV), which is mostly found in patients with AIDS and has poor prognosis. Nuclear factor (NF)-κB pathway is constitutively activated in HHV-8-infected PEL cells and plays a crucial role in tumorigenesis. Recently, it has been shown that diethyldithiocarbamate (DDTC), an active metabolite of disulfiram, has apoptotic activity in cancer cells. Here, we investigated the effect of DDTC on PEL using a PEL mouse model generated by intraperitoneal injection of BC-3 cells, a PEL cell line. DDTC ameliorated the symptoms of PEL in these mice, such as development of ascites, splenomegaly and increase of body weight, in comparison with PBS-treated controls. Moreover, we determined in vitro that DDTC suppressed the constitutively activated NF-κB pathway in BC-3 cells. Methylthiotetrazole assay revealed that the cell proliferation of various PEL cell lines was significantly suppressed by the treatment of DDTC. DDTC also induced the expression of cleaved caspase-3, an apoptosis marker, whereas the addition of Q-VD-OPh, a pan-caspase inhibitor, inhibited cell apoptosis induced by DDTC treatment. Together, our results indicated that DDTC induces apoptosis via inhibition of the NF-κB signaling pathway in HHV-8-infected PEL cells. This study suggests the potential use of DDTC as a therapeutic approach for PEL.
Figures


Similar articles
-
Biscoclaurine alkaloid cepharanthine inhibits the growth of primary effusion lymphoma in vitro and in vivo and induces apoptosis via suppression of the NF-kappaB pathway.Int J Cancer. 2009 Sep 15;125(6):1464-72. doi: 10.1002/ijc.24521. Int J Cancer. 2009. PMID: 19521981
-
A purine scaffold HSP90 inhibitor BIIB021 has selective activity against KSHV-associated primary effusion lymphoma and blocks vFLIP K13-induced NF-κB.Clin Cancer Res. 2013 Sep 15;19(18):5016-26. doi: 10.1158/1078-0432.CCR-12-3510. Epub 2013 Jul 23. Clin Cancer Res. 2013. PMID: 23881928 Free PMC article.
-
Transient inhibition of NF-kappaB by DHMEQ induces cell death of primary effusion lymphoma without HHV-8 reactivation.Cancer Sci. 2009 Apr;100(4):737-46. doi: 10.1111/j.1349-7006.2009.01083.x. Cancer Sci. 2009. PMID: 19469019 Free PMC article.
-
Human Herpesvirus Type 8-associated Large B-cell Lymphoma: A Nonserous Extracavitary Variant of Primary Effusion Lymphoma in an HIV-infected Man: A Case Report and Review of the Literature.Clin Lymphoma Myeloma Leuk. 2016 Jun;16(6):311-21. doi: 10.1016/j.clml.2016.03.013. Epub 2016 Apr 1. Clin Lymphoma Myeloma Leuk. 2016. PMID: 27234438 Free PMC article. Review.
-
Lymphoma cell lines: in vitro models for the study of HHV-8+ primary effusion lymphomas (body cavity-based lymphomas).Leukemia. 1998 Oct;12(10):1507-17. doi: 10.1038/sj.leu.2401160. Leukemia. 1998. PMID: 9766492 Review.
Cited by
-
Diethyldithiocarbamate, an anti-abuse drug, alleviates steatohepatitis and fibrosis in rodents through modulating lipid metabolism and oxidative stress.Br J Pharmacol. 2018 Dec;175(24):4480-4495. doi: 10.1111/bph.14503. Epub 2018 Oct 23. Br J Pharmacol. 2018. PMID: 30266038 Free PMC article.
-
Matrix metalloproteinase-1 induction by diethyldithiocarbamate is regulated via Akt and ERK/miR222/ETS-1 pathways in hepatic stellate cells.Biosci Rep. 2016 Aug 24;36(4):e00371. doi: 10.1042/BSR20160111. Print 2016 Aug. Biosci Rep. 2016. PMID: 27412967 Free PMC article.
-
Role of Pattern Recognition Receptors in KSHV Infection.Cancers (Basel). 2018 Mar 20;10(3):85. doi: 10.3390/cancers10030085. Cancers (Basel). 2018. PMID: 29558453 Free PMC article. Review.
-
Primary effusion lymphoma in an elderly patient effectively treated by lenalidomide: case report and review of literature.Blood Cancer J. 2014 Mar 7;4(3):e190. doi: 10.1038/bcj.2014.6. Blood Cancer J. 2014. PMID: 24608734 Free PMC article.
-
Current status of treatment for primary effusion lymphoma.Intractable Rare Dis Res. 2014 Aug;3(3):65-74. doi: 10.5582/irdr.2014.01010. Intractable Rare Dis Res. 2014. PMID: 25364646 Free PMC article. Review.
References
-
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569–576. - PubMed
-
- Nador RG, Cesarman E, Chadburn A, Dawson DB, Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood. 1996;88:645–656. - PubMed
-
- Boulanger E, Gerard L, Gabarre J, Molina JM, Rapp C, Abino JF, Cadranel J, Chevret S, Oksenhendler E. Prognostic factors and outcome of human herpesvirus 8-associated primary effusion lymphoma in patients with AIDS. J Clin Oncol. 2005;23:4372–4380. - PubMed
-
- An J, Sun Y, Sun R, Rettig MB. Kaposi’s sarcoma-associated herpesvirus encoded vFLIP induces cellular IL-6 expression: the role of the NF-kappaB and JNK/AP1 pathways. Oncogene. 2003;22:3371–3385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

