The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+)
- PMID: 22195961
- PMCID: PMC3331675
- DOI: 10.1016/j.molcel.2011.12.005
The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+)
Abstract
The NAD(+)-dependent deacetylase SIRT1 is an evolutionarily conserved metabolic sensor of the Sirtuin family that mediates homeostatic responses to certain physiological stresses such as nutrient restriction. Previous reports have implicated fluctuations in intracellular NAD(+) concentrations as the principal regulator of SIRT1 activity. However, here we have identified a cAMP-induced phosphorylation of a highly conserved serine (S434) located in the SIRT1 catalytic domain that rapidly enhanced intrinsic deacetylase activity independently of changes in NAD(+) levels. Attenuation of SIRT1 expression or the use of a nonphosphorylatable SIRT1 mutant prevented cAMP-mediated stimulation of fatty acid oxidation and gene expression linked to this pathway. Overexpression of SIRT1 in mice significantly potentiated the increases in fatty acid oxidation and energy expenditure caused by either pharmacological β-adrenergic agonism or cold exposure. These studies support a mechanism of Sirtuin enzymatic control through the cAMP/PKA pathway with important implications for stress responses and maintenance of energy homeostasis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
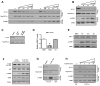
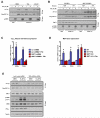

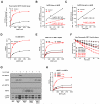
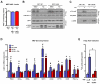
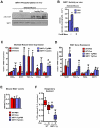
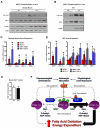
Comment in
-
SIRT1 regulation-it ain't all NAD.Mol Cell. 2012 Jan 13;45(1):9-11. doi: 10.1016/j.molcel.2011.12.017. Mol Cell. 2012. PMID: 22244328
Similar articles
-
Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of SIRT1-PGC1α complex.J Biol Chem. 2013 Mar 8;288(10):7117-26. doi: 10.1074/jbc.M112.415729. Epub 2013 Jan 17. J Biol Chem. 2013. PMID: 23329830 Free PMC article.
-
Activation of SIRT1 by L-serine increases fatty acid oxidation and reverses insulin resistance in C2C12 myotubes.Cell Biol Toxicol. 2019 Oct;35(5):457-470. doi: 10.1007/s10565-019-09463-x. Epub 2019 Feb 5. Cell Biol Toxicol. 2019. PMID: 30721374
-
Activation of type 2 cannabinoid receptors (CB2R) promotes fatty acid oxidation through the SIRT1/PGC-1α pathway.Biochem Biophys Res Commun. 2013 Jul 5;436(3):377-81. doi: 10.1016/j.bbrc.2013.05.108. Epub 2013 Jun 4. Biochem Biophys Res Commun. 2013. PMID: 23747418
-
Role of mitochondria in diabetic peripheral neuropathy: Influencing the NAD+-dependent SIRT1-PGC-1α-TFAM pathway.Int Rev Neurobiol. 2019;145:177-209. doi: 10.1016/bs.irn.2019.04.002. Epub 2019 Jun 8. Int Rev Neurobiol. 2019. PMID: 31208524 Free PMC article. Review.
-
Metabolic benefits from Sirt1 and Sirt1 activators.Curr Opin Clin Nutr Metab Care. 2009 Jul;12(4):431-7. doi: 10.1097/MCO.0b013e32832cdaae. Curr Opin Clin Nutr Metab Care. 2009. PMID: 19474719 Review.
Cited by
-
Nuclear Perilipin 5 integrates lipid droplet lipolysis with PGC-1α/SIRT1-dependent transcriptional regulation of mitochondrial function.Nat Commun. 2016 Aug 24;7:12723. doi: 10.1038/ncomms12723. Nat Commun. 2016. PMID: 27554864 Free PMC article.
-
Metabolic regulation by SIRT3: implications for tumorigenesis.Trends Mol Med. 2012 Sep;18(9):516-23. doi: 10.1016/j.molmed.2012.05.004. Epub 2012 Jun 27. Trends Mol Med. 2012. PMID: 22749020 Free PMC article. Review.
-
Mitochondrial metabolism supports resistance to IDH mutant inhibitors in acute myeloid leukemia.J Exp Med. 2021 May 3;218(5):e20200924. doi: 10.1084/jem.20200924. J Exp Med. 2021. PMID: 33760042 Free PMC article.
-
NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise.Am J Physiol Endocrinol Metab. 2012 Aug 1;303(3):E308-21. doi: 10.1152/ajpendo.00054.2012. Epub 2012 Mar 20. Am J Physiol Endocrinol Metab. 2012. PMID: 22436696 Free PMC article. Review.
-
ATGL-catalyzed lipolysis regulates SIRT1 to control PGC-1α/PPAR-α signaling.Diabetes. 2015 Feb;64(2):418-26. doi: 10.2337/db14-0325. Diabetes. 2015. PMID: 25614670 Free PMC article.
References
-
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. - PubMed
-
- Beausoleil SA, Villén J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006;24:1285–1292. - PubMed
-
- Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

