Cancer immunotherapy comes of age
- PMID: 22193102
- PMCID: PMC3967235
- DOI: 10.1038/nature10673
Cancer immunotherapy comes of age
Abstract
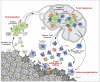
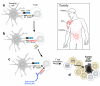
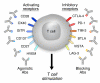
Activating the immune system for therapeutic benefit in cancer has long been a goal in immunology and oncology. After decades of disappointment, the tide has finally changed due to the success of recent proof-of-concept clinical trials. Most notable has been the ability of the anti-CTLA4 antibody, ipilimumab, to achieve a significant increase in survival for patients with metastatic melanoma, for which conventional therapies have failed. In the context of advances in the understanding of how tolerance, immunity and immunosuppression regulate antitumour immune responses together with the advent of targeted therapies, these successes suggest that active immunotherapy represents a path to obtain a durable and long-lasting response in cancer patients.
Figures
Similar articles
-
Prostate cancer immunotherapy.Clin Cancer Res. 2011 Aug 15;17(16):5233-8. doi: 10.1158/1078-0432.CCR-10-3402. Epub 2011 Jun 23. Clin Cancer Res. 2011. PMID: 21700764 Free PMC article.
-
Immunotherapy and therapeutic vaccines in prostate cancer: an update on current strategies and clinical implications.Asian J Androl. 2014 May-Jun;16(3):364-71. doi: 10.4103/1008-682X.122585. Asian J Androl. 2014. PMID: 24435055 Free PMC article. Review.
-
Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types.Clin Cancer Res. 2013 Mar 1;19(5):1009-20. doi: 10.1158/1078-0432.CCR-12-2982. Clin Cancer Res. 2013. PMID: 23460532
-
Combining active immunotherapy with immune checkpoint blockade for the treatment of advanced prostate cancer.Asian J Androl. 2012 Jul;14(4):520-1. doi: 10.1038/aja.2012.45. Epub 2012 May 14. Asian J Androl. 2012. PMID: 22580638 Free PMC article. No abstract available.
-
Molecular insights into the development of T cell-based immunotherapy for prostate cancer.Expert Rev Clin Immunol. 2014 Nov;10(11):1547-57. doi: 10.1586/1744666X.2014.962515. Epub 2014 Sep 26. Expert Rev Clin Immunol. 2014. PMID: 25259804 Review.
Cited by
-
Guiding principles in the design of molecular bioconjugates for vaccine applications.Bioconjug Chem. 2015 May 20;26(5):791-801. doi: 10.1021/acs.bioconjchem.5b00103. Epub 2015 Apr 16. Bioconjug Chem. 2015. PMID: 25822926 Free PMC article. Review.
-
Functional DNA demethylation is accompanied by chromatin accessibility.Nucleic Acids Res. 2013 Apr;41(7):3973-85. doi: 10.1093/nar/gkt077. Epub 2013 Feb 13. Nucleic Acids Res. 2013. PMID: 23408854 Free PMC article.
-
Radiation Dose Escalation is Crucial in Anti-CTLA-4 Antibody Therapy to Enhance Local and Distant Antitumor Effect in Murine Osteosarcoma.Cancers (Basel). 2020 Jun 12;12(6):1546. doi: 10.3390/cancers12061546. Cancers (Basel). 2020. PMID: 32545427 Free PMC article.
-
Novel antigens in non-small cell lung cancer: SP17, AKAP4, and PTTG1 are potential immunotherapeutic targets.Oncotarget. 2015 Feb 20;6(5):2812-26. doi: 10.18632/oncotarget.2802. Oncotarget. 2015. PMID: 25739119 Free PMC article.
-
Targeting Innate Immunity in Glioma Therapy.Int J Mol Sci. 2024 Jan 12;25(2):947. doi: 10.3390/ijms25020947. Int J Mol Sci. 2024. PMID: 38256021 Free PMC article. Review.
References
-
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi:10.1146/annurev.immunol.021908.132544. - PubMed
-
- Hall SS. A commotion in the blood: life, death, and the immune system. Henry Holt; 1997.
-
- Sylvester RJ. Bacillus Calmette-Guerin treatment of non-muscle invasive bladder cancer. Int J Urol. 2011;18:113–120. doi:10.1111/j.1442-2042.2010.02678.x. - PubMed
-
- Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi:10.1146/annurev.immunol.24.021605.090733. - PubMed
-
- Segal NH, et al. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi:10.1158/0008-5472.CAN-07-3095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

