Temporal control of gene deletion in sensory ganglia using a tamoxifen-inducible Advillin-Cre-ERT2 recombinase mouse
- PMID: 22188729
- PMCID: PMC3260248
- DOI: 10.1186/1744-8069-7-100
Temporal control of gene deletion in sensory ganglia using a tamoxifen-inducible Advillin-Cre-ERT2 recombinase mouse
Abstract
Background: Tissue-specific gene deletion has proved informative in the analysis of pain pathways. Advillin has been shown to be a pan-neuronal marker of spinal and cranial sensory ganglia. We generated BAC transgenic mice using the Advillin promoter to drive a tamoxifen-inducible CreERT2 recombinase construct in order to be able to delete genes in adult animals. We used a floxed stop ROSA26LacZ reporter mouse to examine functional Cre expression, and analysed the behaviour of mice expressing Cre recombinase.
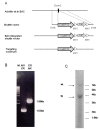
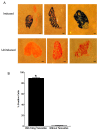
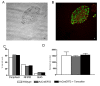
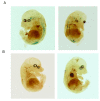
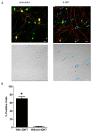
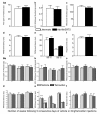
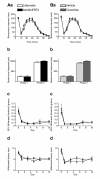
Results: We used recombineering to introduce a CreERT2 cassette in place of exon 2 of the Advillin gene into a BAC clone (RPCI23-424F19) containing the 5' region of the Advillin gene. Transgenic mice were generated using pronuclear injection. The resulting AvCreERT2 transgenic mice showed a highly specific expression pattern of Cre activity after tamoxifen induction. Recombinase activity was confined to sensory neurons and no expression was found in other organs. Less than 1% of neurons showed Cre expression in the absence of tamoxifen treatment. Five-day intraperitoneal treatment with tamoxifen (2 mg per day) induced Cre recombination events in ≈90% of neurons in dorsal root and cranial ganglia. Cell counts of dorsal root ganglia (DRG) from transgenic animals with or without tamoxifen treatment showed no neuronal cell loss. Sensory neurons in culture showed ≈70% induction after 3 days treatment with tamoxifen. Behavioural tests showed no differences between wildtype, AvCreERT2 and tamoxifen-treated animals in terms of motor function, responses to light touch and noxious pressure, thermal thresholds as well as responses to inflammatory agents.
Conclusions: Our results suggest that the inducible pan-DRG AvCreERT2 deleter mouse strain is a useful tool for studying the role of individual genes in adult sensory neuron function. The pain phenotype of the Cre-induced animal is normal; therefore any alterations in pain processing can be unambiguously attributed to loss of the targeted gene.
Figures
Similar articles
-
Tamoxifen-inducible NaV1.8-CreERT2 recombinase activity in nociceptive neurons of dorsal root ganglia.Genesis. 2006 Aug;44(8):364-71. doi: 10.1002/dvg.20224. Genesis. 2006. PMID: 16850455
-
Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2.Glia. 2006 Jul;54(1):11-20. doi: 10.1002/glia.20342. Glia. 2006. PMID: 16575885
-
Conditional deletion of Pip5k1c in sensory ganglia and effects on nociception and inflammatory sensitization.Mol Pain. 2017 Jan-Dec;13:1744806917737907. doi: 10.1177/1744806917737907. Mol Pain. 2017. PMID: 29020859 Free PMC article.
-
Endothelial-Specific Cre Mouse Models.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2550-2561. doi: 10.1161/ATVBAHA.118.309669. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354251 Free PMC article. Review.
-
Tools and Techniques for Wt1-Based Lineage Tracing.Methods Mol Biol. 2016;1467:41-59. doi: 10.1007/978-1-4939-4023-3_4. Methods Mol Biol. 2016. PMID: 27417958 Review.
Cited by
-
Anthrax toxins regulate pain signaling and can deliver molecular cargoes into ANTXR2+ DRG sensory neurons.Nat Neurosci. 2022 Feb;25(2):168-179. doi: 10.1038/s41593-021-00973-8. Epub 2021 Dec 20. Nat Neurosci. 2022. PMID: 34931070 Free PMC article.
-
Pain without nociceptors? Nav1.7-independent pain mechanisms.Cell Rep. 2014 Jan 30;6(2):301-12. doi: 10.1016/j.celrep.2013.12.033. Epub 2014 Jan 16. Cell Rep. 2014. PMID: 24440715 Free PMC article.
-
NMDA Receptors at Primary Afferent-Excitatory Neuron Synapses Differentially Sustain Chemotherapy- and Nerve Trauma-Induced Chronic Pain.J Neurosci. 2023 May 24;43(21):3933-3948. doi: 10.1523/JNEUROSCI.0183-23.2023. Epub 2023 Apr 26. J Neurosci. 2023. PMID: 37185237 Free PMC article.
-
Sensory nerves in the spotlight of the stem cell niche.Stem Cells Transl Med. 2021 Mar;10(3):346-356. doi: 10.1002/sctm.20-0284. Epub 2020 Oct 28. Stem Cells Transl Med. 2021. PMID: 33112056 Free PMC article. Review.
-
Manipulating the Mouse Genome Using Recombineering.Adv Genet Eng. 2013;2(2):108. doi: 10.4172/2169-0111.1000108. Epub 2013 Jun 27. Adv Genet Eng. 2013. PMID: 31404315 Free PMC article.
References
-
- Lacroix-Fralish ML, Ledoux JB, Mogil JS. The Pain Genes Database: An interactive web browser of pain-related transgenic knockout studies. Pain. 2007;131:3–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

