A complex of Protocadherin-19 and N-cadherin mediates a novel mechanism of cell adhesion
- PMID: 22184198
- PMCID: PMC3246890
- DOI: 10.1083/jcb.201108115
A complex of Protocadherin-19 and N-cadherin mediates a novel mechanism of cell adhesion
Abstract
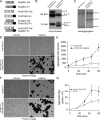
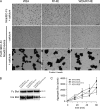
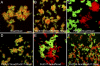
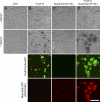
During embryonic morphogenesis, adhesion molecules are required for selective cell-cell interactions. The classical cadherins mediate homophilic calcium-dependent cell adhesion and are founding members of the large and diverse cadherin superfamily. The protocadherins are the largest subgroup within this superfamily, yet their participation in calcium-dependent cell adhesion is uncertain. In this paper, we demonstrate a novel mechanism of adhesion, mediated by a complex of Protocadherin-19 (Pcdh19) and N-cadherin (Ncad). Although Pcdh19 alone is only weakly adhesive, the Pcdh19-Ncad complex exhibited robust adhesion in bead aggregation assays, and Pcdh19 appeared to play the dominant role. Adhesion by the Pcdh19-Ncad complex was unaffected by mutations that disrupt Ncad homophilic binding but was inhibited by a mutation in Pcdh19. In addition, the complex exhibited homophilic specificity, as beads coated with Pcdh19-Ncad did not intermix with Ncad- or Pcdh17-Ncad-coated beads. We propose a model in which association of a protocadherin with Ncad acts as a switch, converting between distinct binding specificities.
Figures

Similar articles
-
Zebrafish calsyntenins mediate homophilic adhesion through their amino-terminal cadherin repeats.Neuroscience. 2015 Feb 12;286:87-96. doi: 10.1016/j.neuroscience.2014.11.030. Epub 2014 Nov 26. Neuroscience. 2015. PMID: 25463516 Free PMC article.
-
Structural determinants of adhesion by Protocadherin-19 and implications for its role in epilepsy.Elife. 2016 Oct 26;5:e18529. doi: 10.7554/eLife.18529. Elife. 2016. PMID: 27787195 Free PMC article.
-
Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation.J Cell Biol. 2010 Nov 29;191(5):1029-41. doi: 10.1083/jcb.201007008. J Cell Biol. 2010. PMID: 21115806 Free PMC article.
-
Non-clustered protocadherin.Cell Adh Migr. 2011 Mar-Apr;5(2):97-105. doi: 10.4161/cam.5.2.14374. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21173574 Free PMC article. Review.
-
Analysis of desmosomal cadherin-adhesive function and stoichiometry of desmosomal cadherin-plakoglobin complexes.J Invest Dermatol. 1996 Sep;107(3):293-300. doi: 10.1111/1523-1747.ep12363000. J Invest Dermatol. 1996. PMID: 8751959 Review.
Cited by
-
Protocadherins, not prototypical: a complex tale of their interactions, expression, and functions.Front Mol Neurosci. 2013 Mar 19;6:4. doi: 10.3389/fnmol.2013.00004. eCollection 2013. Front Mol Neurosci. 2013. PMID: 23515683 Free PMC article.
-
Ethanol Effects on Early Developmental Stages Studied Using the Zebrafish.Biomedicines. 2022 Oct 13;10(10):2555. doi: 10.3390/biomedicines10102555. Biomedicines. 2022. PMID: 36289818 Free PMC article. Review.
-
The clustered protocadherins Pcdhα and Pcdhγ form a heteromeric complex in zebrafish.Neuroscience. 2012 Sep 6;219:280-9. doi: 10.1016/j.neuroscience.2012.05.058. Epub 2012 Jun 1. Neuroscience. 2012. PMID: 22659564 Free PMC article.
-
Zebrafish calsyntenins mediate homophilic adhesion through their amino-terminal cadherin repeats.Neuroscience. 2015 Feb 12;286:87-96. doi: 10.1016/j.neuroscience.2014.11.030. Epub 2014 Nov 26. Neuroscience. 2015. PMID: 25463516 Free PMC article.
-
Spatio-temporally restricted expression of cell adhesion molecules during chicken embryonic development.PLoS One. 2014 May 7;9(5):e96837. doi: 10.1371/journal.pone.0096837. eCollection 2014. PLoS One. 2014. PMID: 24806091 Free PMC article.
References
-
- Depienne C., Bouteiller D., Keren B., Cheuret E., Poirier K., Trouillard O., Benyahia B., Quelin C., Carpentier W., Julia S., et al. 2009. Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet. 5:e1000381 10.1371/journal.pgen.1000381 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

