The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-α receptor
- PMID: 22179202
- PMCID: PMC3647613
- DOI: 10.1038/ni.2193
The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-α receptor
Abstract
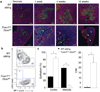
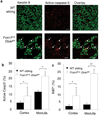
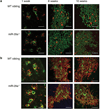
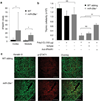
Thymic output is a dynamic process, with high activity at birth punctuated by transient periods of involution during infection. Interferon-α (IFN-α) is a critical molecular mediator of pathogen-induced thymic involution, yet despite the importance of thymic involution, relatively little is known about the molecular integrators that establish sensitivity. Here we found that the microRNA network dependent on the endoribonuclease Dicer, and specifically microRNA miR-29a, was critical for diminishing the sensitivity of the thymic epithelium to simulated infection signals, protecting the thymus against inappropriate involution. In the absence of Dicer or the miR-29a cluster in the thymic epithelium, expression of the IFN-α receptor by the thymic epithelium was higher, which allowed suboptimal signals to trigger rapid loss of thymic cellularity.
Figures




Similar articles
-
Activation of melanoma differentiation-associated gene 5 causes rapid involution of the thymus.J Immunol. 2009 May 15;182(10):6044-50. doi: 10.4049/jimmunol.0803809. J Immunol. 2009. PMID: 19414755
-
Decreased expression of miR-29 family associated with autoimmune myasthenia gravis.J Neuroinflammation. 2020 Oct 8;17(1):294. doi: 10.1186/s12974-020-01958-3. J Neuroinflammation. 2020. PMID: 33032631 Free PMC article.
-
MicroRNA Functions in Thymic Biology: Thymic Development and Involution.Front Immunol. 2018 Sep 11;9:2063. doi: 10.3389/fimmu.2018.02063. eCollection 2018. Front Immunol. 2018. PMID: 30254640 Free PMC article. Review.
-
Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue.Eur J Immunol. 2012 May;42(5):1073-9. doi: 10.1002/eji.201142305. Eur J Immunol. 2012. PMID: 22539280 Review.
-
The modulation of Dicer regulates tumor immunogenicity in melanoma.Oncotarget. 2016 Jul 26;7(30):47663-47673. doi: 10.18632/oncotarget.10273. Oncotarget. 2016. PMID: 27356752 Free PMC article.
Cited by
-
Premature thymic involution is independent of structural plasticity of the thymic stroma.Eur J Immunol. 2015 May;45(5):1535-47. doi: 10.1002/eji.201445277. Epub 2015 Feb 23. Eur J Immunol. 2015. PMID: 25627671 Free PMC article.
-
Molecular and cellular mechanisms of Mycobacterium avium-induced thymic atrophy.J Immunol. 2012 Oct 1;189(7):3600-8. doi: 10.4049/jimmunol.1201525. Epub 2012 Aug 24. J Immunol. 2012. PMID: 22922815 Free PMC article.
-
Age-related thymic involution: Mechanisms and functional impact.Aging Cell. 2022 Aug;21(8):e13671. doi: 10.1111/acel.13671. Epub 2022 Jul 12. Aging Cell. 2022. PMID: 35822239 Free PMC article. Review.
-
Dgcr8 knockout approaches to understand microRNA functions in vitro and in vivo.Cell Mol Life Sci. 2019 May;76(9):1697-1711. doi: 10.1007/s00018-019-03020-9. Epub 2019 Jan 29. Cell Mol Life Sci. 2019. PMID: 30694346 Free PMC article. Review.
-
Pax5 regulates B cell immunity by promoting PI3K signaling via PTEN down-regulation.Sci Immunol. 2021 Jul 23;6(61):eabg5003. doi: 10.1126/sciimmunol.abg5003. Sci Immunol. 2021. PMID: 34301800 Free PMC article.
References
-
- Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–749. - PubMed
-
- Itoh M, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. - PubMed
-
- Anz D, et al. Activation of melanoma differentiation-associated gene 5 causes rapid involution of the thymus. J Immunol. 2009;182:6044–6050. - PubMed
-
- Aronson M. Hypothesis: involution of the thymus with aging--programmed and beneficial. Thymus. 1991;18:7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

