Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex
- PMID: 22172676
- PMCID: PMC3241923
- DOI: 10.1016/j.devcel.2011.10.009
Myosin V transports secretory vesicles via a Rab GTPase cascade and interaction with the exocyst complex
Abstract
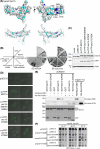
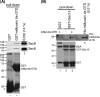
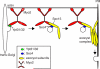
Vesicle transport requires four steps: vesicle formation, movement, tethering, and fusion. In yeast, two Rab GTPases, Ypt31/32, are required for post-Golgi vesicle formation. A third Rab GTPase, Sec4, and the exocyst act in tethering and fusion of these vesicles. Vesicle production is coupled to transport via direct interaction between Ypt31/32 and the yeast myosin V, Myo2. Here we show that Myo2 interacts directly with Sec4 and the exocyst subunit Sec15. Disruption of these interactions results in compromised growth and the accumulation of secretory vesicles. We identified the Sec15-binding region on Myo2 and also identified residues on Sec15 required for interaction with Myo2. That Myo2 interacts with Sec15 uncovers additional roles for the exocyst as an adaptor for molecular motors and implies similar roles for structurally related tethering complexes. Moreover, these studies predict that for many pathways, molecular motors attach to vesicles prior to their formation and remain attached until fusion.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Yeast homologues of lethal giant larvae and type V myosin cooperate in the regulation of Rab-dependent vesicle clustering and polarized exocytosis.Mol Biol Cell. 2011 Mar 15;22(6):842-57. doi: 10.1091/mbc.E10-07-0570. Epub 2011 Jan 19. Mol Biol Cell. 2011. PMID: 21248204 Free PMC article.
-
Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion.Mol Biol Cell. 2008 Oct;19(10):4177-87. doi: 10.1091/mbc.e08-02-0220. Epub 2008 Jul 23. Mol Biol Cell. 2008. PMID: 18653471 Free PMC article.
-
Kinesin-related Smy1 enhances the Rab-dependent association of myosin-V with secretory cargo.Mol Biol Cell. 2016 Aug 1;27(15):2450-62. doi: 10.1091/mbc.E16-03-0185. Epub 2016 Jun 15. Mol Biol Cell. 2016. PMID: 27307583 Free PMC article.
-
The Exocyst at a Glance.J Cell Sci. 2015 Aug 15;128(16):2957-64. doi: 10.1242/jcs.156398. Epub 2015 Aug 3. J Cell Sci. 2015. PMID: 26240175 Free PMC article. Review.
-
The exocyst complex in polarized exocytosis.Int Rev Cytol. 2004;233:243-65. doi: 10.1016/S0074-7696(04)33006-8. Int Rev Cytol. 2004. PMID: 15037366 Review.
Cited by
-
Asymmetric tethering by exocyst in vitro requires a Rab GTPase, an R-SNARE and a Sac1-sensitive phosphoinositide lipid.Mol Biol Cell. 2024 Mar 1;35(3):br8. doi: 10.1091/mbc.E23-08-0311. Epub 2024 Jan 10. Mol Biol Cell. 2024. PMID: 38198574 Free PMC article.
-
Leading the way out: how a plant myosin facilitates vesicle tethering during exocytosis.Plant Cell. 2021 Aug 13;33(7):2104-2105. doi: 10.1093/plcell/koab111. Plant Cell. 2021. PMID: 35233601 Free PMC article. No abstract available.
-
Structural basis of myosin V Rab GTPase-dependent cargo recognition.Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20443-8. doi: 10.1073/pnas.1314329110. Epub 2013 Nov 18. Proc Natl Acad Sci U S A. 2013. PMID: 24248336 Free PMC article.
-
The class V myosin interactome of the human pathogen Aspergillus fumigatus reveals novel interactions with COPII vesicle transport proteins.Biochem Biophys Res Commun. 2020 Jun 18;527(1):232-237. doi: 10.1016/j.bbrc.2020.04.111. Epub 2020 May 1. Biochem Biophys Res Commun. 2020. PMID: 32446373 Free PMC article.
-
The role of Munc18-1 and its orthologs in modulation of cortical F-actin in chromaffin cells.J Mol Neurosci. 2012 Oct;48(2):339-46. doi: 10.1007/s12031-012-9775-8. Epub 2012 Apr 26. J Mol Neurosci. 2012. PMID: 22535313 Free PMC article. Review.
References
-
- Brennwald P, Kearns B, Champion K, Keranen S, Bankaitis V, Novick P. Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell. 1994;79:245–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

