Constitutive upregulation of chaperone-mediated autophagy in Huntington's disease
- PMID: 22171050
- PMCID: PMC3282924
- DOI: 10.1523/JNEUROSCI.3219-11.2011
Constitutive upregulation of chaperone-mediated autophagy in Huntington's disease
Abstract
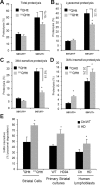
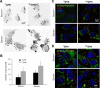
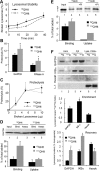
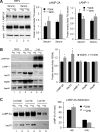
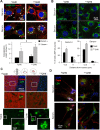
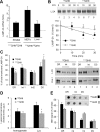
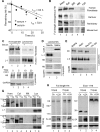
Autophagy contributes to the removal of prone-to-aggregate proteins, but in several instances these pathogenic proteins have been shown to interfere with autophagic activity. In the case of Huntington's disease (HD), a congenital neurodegenerative disorder resulting from mutation in the huntingtin protein, we have previously described that the mutant protein interferes with the ability of autophagic vacuoles to recognize cytosolic cargo. Growing evidence supports the existence of cross talk among autophagic pathways, suggesting the possibility of functional compensation when one of them is compromised. In this study, we have identified a compensatory upregulation of chaperone-mediated autophagy (CMA) in different cellular and mouse models of HD. Components of CMA, namely the lysosome-associated membrane protein type 2A (LAMP-2A) and lysosomal-hsc70, are markedly increased in HD models. The increase in LAMP-2A is achieved through both an increase in the stability of this protein at the lysosomal membrane and transcriptional upregulation of this splice variant of the lamp-2 gene. We propose that CMA activity increases in response to macroautophagic dysfunction in the early stages of HD, but that the efficiency of this compensatory mechanism may decrease with age and so contribute to cellular failure and the onset of pathological manifestations.
Figures

Similar articles
-
Role of chaperone-mediated autophagy in degrading Huntington's disease-associated huntingtin protein.Acta Biochim Biophys Sin (Shanghai). 2014 Feb;46(2):83-91. doi: 10.1093/abbs/gmt133. Epub 2013 Dec 8. Acta Biochim Biophys Sin (Shanghai). 2014. PMID: 24323530 Review.
-
The role of chaperone-mediated autophagy in huntingtin degradation.PLoS One. 2012;7(10):e46834. doi: 10.1371/journal.pone.0046834. Epub 2012 Oct 11. PLoS One. 2012. PMID: 23071649 Free PMC article.
-
The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane.Mol Cell Biol. 2008 Sep;28(18):5747-63. doi: 10.1128/MCB.02070-07. Epub 2008 Jul 21. Mol Cell Biol. 2008. PMID: 18644871 Free PMC article.
-
Molecular control of chaperone-mediated autophagy.Essays Biochem. 2017 Dec 12;61(6):663-674. doi: 10.1042/EBC20170057. Print 2017 Dec 12. Essays Biochem. 2017. PMID: 29233876 Review.
-
Structure of transmembrane domain of lysosome-associated membrane protein type 2a (LAMP-2A) reveals key features for substrate specificity in chaperone-mediated autophagy.J Biol Chem. 2014 Dec 19;289(51):35111-23. doi: 10.1074/jbc.M114.609446. Epub 2014 Oct 22. J Biol Chem. 2014. PMID: 25342746 Free PMC article.
Cited by
-
Targeting autophagy in neurodegenerative diseases: From molecular mechanisms to clinical therapeutics.Clin Exp Pharmacol Physiol. 2021 Jul;48(7):943-953. doi: 10.1111/1440-1681.13500. Epub 2021 Apr 21. Clin Exp Pharmacol Physiol. 2021. PMID: 33752254 Free PMC article. Review.
-
Nitric oxide contributes to protein homeostasis by S-nitrosylations of the chaperone HSPA8 and the ubiquitin ligase UBE2D.Redox Biol. 2019 Jan;20:217-235. doi: 10.1016/j.redox.2018.10.002. Epub 2018 Oct 16. Redox Biol. 2019. PMID: 30368041 Free PMC article.
-
Dysfunction of chaperone-mediated autophagy in human diseases.Mol Cell Biochem. 2021 Mar;476(3):1439-1454. doi: 10.1007/s11010-020-04006-z. Epub 2021 Jan 3. Mol Cell Biochem. 2021. PMID: 33389491 Review.
-
Spatiotemporal analysis of soluble aggregates and autophagy markers in the R6/2 mouse model.Sci Rep. 2021 Jan 8;11(1):96. doi: 10.1038/s41598-020-78850-w. Sci Rep. 2021. PMID: 33420088 Free PMC article.
-
Legal but lethal: functional protein aggregation at the verge of toxicity.Front Cell Neurosci. 2015 Feb 18;9:45. doi: 10.3389/fncel.2015.00045. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25741240 Free PMC article. Review.
References
-
- Aniento F, Roche E, Cuervo AM, Knecht E. Uptake and degradation of glyceraldehyde-3-phosphate dehydrogenase by rat liver lysosomes. J Biol Chem. 1993;268:10463–10470. - PubMed
-
- Auteri JS, Okada A, Bochaki V, Dice JF. Regulation of intracellular protein degradation in IMR-90 human diploid fibroblasts. J Cell Physiol. 1983;115:167–174. - PubMed
-
- Bauer PO, Goswami A, Wong HK, Okuno M, Kurosawa M, Yamada M, Miyazaki H, Matsumoto G, Kino Y, Nagai Y, Nukina N. Harnessing chaperone-mediated autophagy for the selective degradation of mutant huntingtin protein. Nat Biotechnol. 2010;28:256–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG021904/AG/NIA NIH HHS/United States
- P01 AG031782-05/AG/NIA NIH HHS/United States
- R37 AG021904/AG/NIA NIH HHS/United States
- R01 AG021904-09/AG/NIA NIH HHS/United States
- P30 AG038072/AG/NIA NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- P50 NS038370-14/NS/NINDS NIH HHS/United States
- P50 NS038370-05/NS/NINDS NIH HHS/United States
- P30 AG038072-03/AG/NIA NIH HHS/United States
- T32 AG023475-09/AG/NIA NIH HHS/United States
- T32 AG023475/AG/NIA NIH HHS/United States
- AG031782/AG/NIA NIH HHS/United States
- P01 AG031782/AG/NIA NIH HHS/United States
- AG021904/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

