Inhibition of interferon induction and action by the nairovirus Nairobi sheep disease virus/Ganjam virus
- PMID: 22163042
- PMCID: PMC3230622
- DOI: 10.1371/journal.pone.0028594
Inhibition of interferon induction and action by the nairovirus Nairobi sheep disease virus/Ganjam virus
Abstract
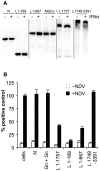
The Nairoviruses are an important group of tick-borne viruses that includes pathogens of man (Crimean Congo hemorrhagic fever virus) and livestock animals (Dugbe virus, Nairobi sheep disease virus (NSDV)). NSDV is found in large parts of East Africa and the Indian subcontinent (where it is known as Ganjam virus). We have investigated the ability of NSDV to antagonise the induction and actions of interferon. Both pathogenic and apathogenic isolates could actively inhibit the induction of type 1 interferon, and also blocked the signalling pathways of both type 1 and type 2 interferons. Using transient expression of viral proteins or sections of viral proteins, these activities all mapped to the ovarian tumour-like protease domain (OTU) found in the viral RNA polymerase. Virus infection, or expression of this OTU domain in transfected cells, led to a great reduction in the incorporation of ubiquitin or ISG15 protein into host cell proteins. Point mutations in the OTU that inhibited the protease activity also prevented it from antagonising interferon induction and action. Interestingly, a mutation at a peripheral site, which had little apparent effect on the ability of the OTU to inhibit ubiquitination and ISG15ylation, removed the ability of the OTU to block the induction of type 1 and the action of type 2 interferons, but had a lesser effect on the ability to block type 1 interferon action, suggesting that targets other than ubiquitin and ISG15 may be involved in the actions of the viral OTU.
Conflict of interest statement
Figures
Similar articles
-
ISG15 overexpression compensates the defect of Crimean-Congo hemorrhagic fever virus polymerase bearing a protease-inactive ovarian tumor domain.PLoS Negl Trop Dis. 2020 Sep 15;14(9):e0008610. doi: 10.1371/journal.pntd.0008610. eCollection 2020 Sep. PLoS Negl Trop Dis. 2020. PMID: 32931521 Free PMC article.
-
The nairovirus nairobi sheep disease virus/ganjam virus induces the translocation of protein disulphide isomerase-like oxidoreductases from the endoplasmic reticulum to the cell surface and the extracellular space.PLoS One. 2014 Apr 8;9(4):e94656. doi: 10.1371/journal.pone.0094656. eCollection 2014. PLoS One. 2014. PMID: 24714576 Free PMC article.
-
Antibodies to the core proteins of Nairobi sheep disease virus/Ganjam virus reveal details of the distribution of the proteins in infected cells and tissues.PLoS One. 2015 Apr 23;10(4):e0124966. doi: 10.1371/journal.pone.0124966. eCollection 2015. PLoS One. 2015. PMID: 25905707 Free PMC article.
-
Ganjam virus.Indian J Med Res. 2009 Nov;130(5):514-9. Indian J Med Res. 2009. PMID: 20090098 Review.
-
The molecular biology of nairoviruses, an emerging group of tick-borne arboviruses.Arch Virol. 2014 Jun;159(6):1249-65. doi: 10.1007/s00705-013-1940-z. Epub 2013 Dec 11. Arch Virol. 2014. PMID: 24327094 Free PMC article. Review.
Cited by
-
ISG15 overexpression compensates the defect of Crimean-Congo hemorrhagic fever virus polymerase bearing a protease-inactive ovarian tumor domain.PLoS Negl Trop Dis. 2020 Sep 15;14(9):e0008610. doi: 10.1371/journal.pntd.0008610. eCollection 2020 Sep. PLoS Negl Trop Dis. 2020. PMID: 32931521 Free PMC article.
-
Morbillivirus v proteins exhibit multiple mechanisms to block type 1 and type 2 interferon signalling pathways.PLoS One. 2013;8(2):e57063. doi: 10.1371/journal.pone.0057063. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431397 Free PMC article.
-
The nairovirus nairobi sheep disease virus/ganjam virus induces the translocation of protein disulphide isomerase-like oxidoreductases from the endoplasmic reticulum to the cell surface and the extracellular space.PLoS One. 2014 Apr 8;9(4):e94656. doi: 10.1371/journal.pone.0094656. eCollection 2014. PLoS One. 2014. PMID: 24714576 Free PMC article.
-
Control of the induction of type I interferon by Peste des petits ruminants virus.PLoS One. 2017 May 5;12(5):e0177300. doi: 10.1371/journal.pone.0177300. eCollection 2017. PLoS One. 2017. PMID: 28475628 Free PMC article.
-
Host Cell Restriction Factors of Bunyaviruses and Viral Countermeasures.Viruses. 2021 Apr 28;13(5):784. doi: 10.3390/v13050784. Viruses. 2021. PMID: 33925004 Free PMC article. Review.
References
-
- Montgomery E. On a tick-borne gastro-enteritis of sheep and goats occuring in British East Africa. J Comp Pathol. 1917;30:28–57.
-
- Marczinke BI, Nichol ST. Nairobi sheep disease virus, an important tick-borne pathogen of sheep and goats in Africa, is also present in Asia. Virology. 2002;303:146–151. - PubMed
-
- Daubney R, Hudson JR. Nairobi Sheep Disease. Parasitology. 1931;23:507–524.
-
- Daubney R, Hudson JR. Nairobi Sheep Disease; natural and experimental transmission by ticks other than Rhipicephalus appendiculatus. Parasitology. 1934;26:496–509.
-
- Dandawate CN, Shah KV. Ganjam virus: a new arbovirus isolated from ticks Haemaphysalis intermedia Warburton and Nuttall, 1909 in Orissa, India. Indian J Med Res. 1969;57:799–804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F006764/2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001433/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F00740X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F006764/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

