Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR)
- PMID: 22160693
- PMCID: PMC3248484
- DOI: 10.1073/pnas.1109773109
Imaging dynamic insulin release using a fluorescent zinc indicator for monitoring induced exocytotic release (ZIMIR)
Abstract
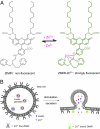
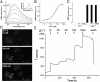
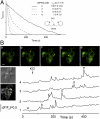
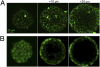
Current methods of monitoring insulin secretion lack the required spatial and temporal resolution to adequately map the dynamics of exocytosis of native insulin granules in intact cell populations in three dimensions. Exploiting the fact that insulin granules contain a high level of Zn(2+), and that Zn(2+) is coreleased with insulin during secretion, we have developed a fluorescent, cell surface-targeted zinc indicator for monitoring induced exocytotic release (ZIMIR). ZIMIR displayed a robust fluorescence enhancement on Zn(2+) chelation and bound Zn(2+) with high selectivity against Ca(2+) and Mg(2+). When added to cultured β cells or intact pancreatic islets at low micromolar concentrations, ZIMIR labeled cells rapidly, noninvasively, and stably, and it reliably reported changes in Zn(2+) concentration near the sites of granule fusion with high sensitivity that correlated well with membrane capacitance measurement. Fluorescence imaging of ZIMIR-labeled β cells followed the dynamics of exocytotic activity at subcellular resolution, even when using simple epifluorescence microscopy, and located the chief sites of insulin release to intercellular junctions. Moreover, ZIMIR imaging of intact rat islets revealed that Zn(2+)/insulin release occurred largely in small groups of adjacent β cells, with each forming a "secretory unit." Concurrent imaging of ZIMIR and Fura-2 showed that the amplitude of cytosolic Ca(2+) elevation did not necessarily correlate with insulin secretion activity, suggesting that events downstream of Ca(2+) signaling underlie the cell-cell heterogeneity in insulin release. In addition to studying stimulation-secretion coupling in cells with Zn(2+)-containing granules, ZIMIR may find applications in β-cell engineering and screening for molecules regulating insulin secretion on high-throughput platforms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
GLP-1 Receptor Mediated Targeting of a Fluorescent Zn(2+) Sensor to Beta Cell Surface for Imaging Insulin/Zn(2+) Release.Bioconjug Chem. 2015 Aug 19;26(8):1443-50. doi: 10.1021/acs.bioconjchem.5b00332. Epub 2015 Jul 6. Bioconjug Chem. 2015. PMID: 26121325
-
In Vivo ZIMIR Imaging of Mouse Pancreatic Islet Cells Shows Oscillatory Insulin Secretion.Front Endocrinol (Lausanne). 2021 Mar 9;12:613964. doi: 10.3389/fendo.2021.613964. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33767668 Free PMC article.
-
Genetic targeting of a small fluorescent zinc indicator to cell surface for monitoring zinc secretion.ACS Chem Biol. 2015 Apr 17;10(4):1054-63. doi: 10.1021/cb5007536. Epub 2015 Jan 27. ACS Chem Biol. 2015. PMID: 25572404 Free PMC article.
-
Insulin exocytotic mechanism by imaging technique.J Biochem. 2006 Jul;140(1):1-5. doi: 10.1093/jb/mvj127. J Biochem. 2006. PMID: 16877762 Review.
-
Granule docking and cargo release in pancreatic beta-cells.Biochem Soc Trans. 2008 Jun;36(Pt 3):294-9. doi: 10.1042/BST0360294. Biochem Soc Trans. 2008. PMID: 18481945 Review.
Cited by
-
Exploring pancreatic beta-cell subgroups and their connectivity.Nat Metab. 2024 Nov;6(11):2039-2053. doi: 10.1038/s42255-024-01097-6. Epub 2024 Aug 8. Nat Metab. 2024. PMID: 39117960 Review.
-
Imaging Beta-Cell Function in the Pancreas of Non-Human Primates Using a Zinc-Sensitive MRI Contrast Agent.Front Endocrinol (Lausanne). 2021 May 26;12:641722. doi: 10.3389/fendo.2021.641722. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34122330 Free PMC article.
-
Live-cell imaging of glucose-induced metabolic coupling of β and α cell metabolism in health and type 2 diabetes.Commun Biol. 2021 May 19;4(1):594. doi: 10.1038/s42003-021-02113-1. Commun Biol. 2021. PMID: 34012065 Free PMC article.
-
β-Cell Pathophysiology: A Review of Advanced Optical Microscopy Applications.Int J Mol Sci. 2021 Nov 26;22(23):12820. doi: 10.3390/ijms222312820. Int J Mol Sci. 2021. PMID: 34884624 Free PMC article. Review.
-
ADCY5 couples glucose to insulin secretion in human islets.Diabetes. 2014 Sep;63(9):3009-21. doi: 10.2337/db13-1607. Epub 2014 Apr 16. Diabetes. 2014. PMID: 24740569 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

