Regulatory control or oxidative damage? Proteomic approaches to interrogate the role of cysteine oxidation status in biological processes
- PMID: 22159599
- PMCID: PMC3322581
- DOI: 10.1074/mcp.R111.013037
Regulatory control or oxidative damage? Proteomic approaches to interrogate the role of cysteine oxidation status in biological processes
Abstract
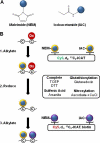
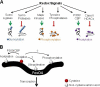
Oxidation is a double-edged sword for cellular processes and its role in normal physiology, cancer and aging remains only partially understood. Although oxidative stress may disrupt biological function, oxidation-reduction (redox) reactions in a cell are often tightly regulated and play essential physiological roles. Cysteines lie at the interface between these extremes since the chemical properties that make specific thiols exquisitely redox-sensitive also predispose them to oxidative damage by reactive oxygen or nitrogen species during stress. Thus, these modifications can be either under reversible redox regulatory control or, alternatively, a result of reversible or irreversible oxidative damage. In either case, it has become increasingly important to assess the redox status of protein thiols since these modifications often impact such processes as catalytic activity, conformational alterations, or metal binding. To better understand the redox changes that accompany protein cysteine residues in complex biological systems, new experimental approaches have been developed to identify and characterize specific thiol modifications and/or changes in their overall redox status. In this review, we describe the recent technologies in redox proteomics that have pushed the boundaries for detecting and quantifying redox cysteine modifications in a cellular context. While there is no one-size-fits-all analytical solution, we highlight the rationale, strengths, and limitations of each technology in order to effectively apply them to specific biological questions. Several technological limitations still remain unsolved, however these approaches and future developments play an important role toward understanding the interplay between oxidative stress and redox signaling in health and disease.
Figures

Similar articles
-
Quantitative redox proteomics: the NOxICAT method.Methods Mol Biol. 2012;893:387-403. doi: 10.1007/978-1-61779-885-6_24. Methods Mol Biol. 2012. PMID: 22665313
-
Proteomic approaches to quantify cysteine reversible modifications in aging and neurodegenerative diseases.Proteomics Clin Appl. 2016 Dec;10(12):1159-1177. doi: 10.1002/prca.201600015. Epub 2016 Nov 11. Proteomics Clin Appl. 2016. PMID: 27666938 Free PMC article. Review.
-
Identification of redox-sensitive cysteines in the Arabidopsis proteome using OxiTRAQ, a quantitative redox proteomics method.Proteomics. 2014 Mar;14(6):750-62. doi: 10.1002/pmic.201300307. Epub 2014 Jan 28. Proteomics. 2014. PMID: 24376095
-
Activity-Based Sensing for Site-Specific Proteomic Analysis of Cysteine Oxidation.Acc Chem Res. 2020 Jan 21;53(1):20-31. doi: 10.1021/acs.accounts.9b00562. Epub 2019 Dec 23. Acc Chem Res. 2020. PMID: 31869209 Free PMC article. Review.
-
Redox proteomics: from bench to bedside.Adv Exp Med Biol. 2014;806:301-17. doi: 10.1007/978-3-319-06068-2_13. Adv Exp Med Biol. 2014. PMID: 24952188 Review.
Cited by
-
Endogenous SO2-dependent Smad3 redox modification controls vascular remodeling.Redox Biol. 2021 May;41:101898. doi: 10.1016/j.redox.2021.101898. Epub 2021 Feb 18. Redox Biol. 2021. PMID: 33647858 Free PMC article.
-
Electrostatics of cysteine residues in proteins: parameterization and validation of a simple model.Proteins. 2012 Nov;80(11):2583-91. doi: 10.1002/prot.24142. Epub 2012 Aug 21. Proteins. 2012. PMID: 22777874 Free PMC article.
-
Mitoproteomics: Tackling Mitochondrial Dysfunction in Human Disease.Oxid Med Cell Longev. 2018 Nov 8;2018:1435934. doi: 10.1155/2018/1435934. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30533169 Free PMC article. Review.
-
Accelerated aging in schizophrenia patients: the potential role of oxidative stress.Aging Dis. 2013 Dec 4;5(4):256-62. doi: 10.14336/AD.2014.0500256. eCollection 2014 Aug. Aging Dis. 2013. PMID: 25110609 Free PMC article. Review.
-
A clickable probe for versatile characterization of S-nitrosothiols.Redox Biol. 2020 Oct;37:101707. doi: 10.1016/j.redox.2020.101707. Epub 2020 Sep 1. Redox Biol. 2020. PMID: 32916549 Free PMC article.
References
-
- Mannick J. B., Hausladen A., Liu L., Hess D. T., Zeng M., Miao Q. X., Kane L. S., Gow A. J., Stamler J. S. (1999) Fas-induced caspase denitrosylation. Science 284, 651–654 - PubMed
-
- Giles N. M., Giles G. I., Jacob C. (2003) Multiple roles of cysteine in biocatalysis. Biochem. Biophys. Res. Commun. 300, 1–4 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

