Protein conformational dynamics in the mechanism of HIV-1 protease catalysis
- PMID: 22158985
- PMCID: PMC3248522
- DOI: 10.1073/pnas.1111202108
Protein conformational dynamics in the mechanism of HIV-1 protease catalysis
Abstract
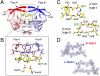
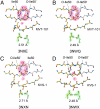
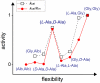
We have used chemical protein synthesis and advanced physical methods to probe dynamics-function correlations for the HIV-1 protease, an enzyme that has received considerable attention as a target for the treatment of AIDS. Chemical synthesis was used to prepare a series of unique analogues of the HIV-1 protease in which the flexibility of the "flap" structures (residues 37-61 in each monomer of the homodimeric protein molecule) was systematically varied. These analogue enzymes were further studied by X-ray crystallography, NMR relaxation, and pulse-EPR methods, in conjunction with molecular dynamics simulations. We show that conformational isomerization in the flaps is correlated with structural reorganization of residues in the active site, and that it is preorganization of the active site that is a rate-limiting factor in catalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Flexibility and function in HIV-1 protease.Nat Struct Biol. 1995 Apr;2(4):274-80. doi: 10.1038/nsb0495-274. Nat Struct Biol. 1995. PMID: 7796263
-
Probing the S1/S1' substrate binding pocket geometry of HIV-1 protease with modified aspartic acid analogues.Biochemistry. 2000 Aug 1;39(30):8768-81. doi: 10.1021/bi000214t. Biochemistry. 2000. PMID: 10913288
-
Curling of flap tips in HIV-1 protease as a mechanism for substrate entry and tolerance of drug resistance.Structure. 2000 Dec 15;8(12):1259-65. doi: 10.1016/s0969-2126(00)00537-2. Structure. 2000. PMID: 11188690
-
Targeting structural flexibility in HIV-1 protease inhibitor binding.Drug Discov Today. 2007 Feb;12(3-4):132-8. doi: 10.1016/j.drudis.2006.12.011. Epub 2006 Dec 20. Drug Discov Today. 2007. PMID: 17275733 Free PMC article. Review.
-
Catalytic contributions from remote regions of enzyme structure.Chem Rev. 2011 Dec 14;111(12):7595-624. doi: 10.1021/cr100042n. Epub 2011 Sep 19. Chem Rev. 2011. PMID: 21923192 Review. No abstract available.
Cited by
-
Binding of the CYK-4 subunit of the centralspindlin complex induces a large scale conformational change in the kinesin subunit.J Biol Chem. 2013 Jul 5;288(27):19785-95. doi: 10.1074/jbc.M113.463695. Epub 2013 May 17. J Biol Chem. 2013. PMID: 23720745 Free PMC article.
-
Small molecule regulation of protein conformation by binding in the Flap of HIV protease.ACS Chem Biol. 2013;8(6):1223-31. doi: 10.1021/cb300611p. Epub 2013 Mar 29. ACS Chem Biol. 2013. PMID: 23540839 Free PMC article.
-
Inhibitor-induced conformational shifts and ligand-exchange dynamics for HIV-1 protease measured by pulsed EPR and NMR spectroscopy.J Phys Chem B. 2012 Dec 13;116(49):14235-44. doi: 10.1021/jp308207h. Epub 2012 Nov 30. J Phys Chem B. 2012. PMID: 23167829 Free PMC article.
-
Parallel Automated Flow Synthesis of Covalent Protein Complexes That Can Inhibit MYC-Driven Transcription.ACS Cent Sci. 2021 Aug 25;7(8):1408-1418. doi: 10.1021/acscentsci.1c00663. Epub 2021 Aug 4. ACS Cent Sci. 2021. PMID: 34471684 Free PMC article.
-
Prediction of HIV-1 protease/inhibitor affinity using RosettaLigand.Chem Biol Drug Des. 2012 Jun;79(6):888-96. doi: 10.1111/j.1747-0285.2012.01356.x. Epub 2012 Mar 19. Chem Biol Drug Des. 2012. PMID: 22321894 Free PMC article.
References
-
- Pomerantz RJ, Horn DL. Twenty years of therapy for HIV-1 infection. Nat Med. 2003;9:867–873. - PubMed
-
- Nicholson LK, et al. Flexibility and function in HIV-1 protease. Nat Struct Biol. 1995;2:274–280. - PubMed
-
- Ishima R, Louis JM. A diverse view of protein dynamics from NMR studies of HIV-1 protease flaps. Proteins. 2008;70:1408–1415. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

