Role of potassium ion channels in detrusor smooth muscle function and dysfunction
- PMID: 22158596
- PMCID: PMC3759241
- DOI: 10.1038/nrurol.2011.194
Role of potassium ion channels in detrusor smooth muscle function and dysfunction
Abstract
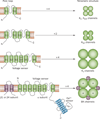
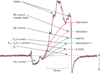
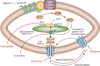
Contraction and relaxation of the detrusor smooth muscle (DSM), which makes up the wall of the urinary bladder, facilitates the storage and voiding of urine. Several families of K(+) channels, including voltage-gated K(+) (K(V)) channels, Ca(2+)-activated K(+) (K(Ca)) channels, inward-rectifying ATP-sensitive K(+) (K(ir), K(ATP)) channels, and two-pore-domain K(+) (K(2P)) channels, are expressed and functional in DSM. They control DSM excitability and contractility by maintaining the resting membrane potential and shaping the action potentials that determine the phasic nature of contractility in this tissue. Defects in DSM K(+) channel proteins or in the molecules involved in their regulatory pathways may underlie certain forms of bladder dysfunction, such as overactive bladder. K(+) channels represent an opportunity for novel pharmacological manipulation and therapeutic intervention in human DSM. Modulation of DSM K(+) channels directly or indirectly by targeting their regulatory mechanisms has the potential to control urinary bladder function. This Review summarizes our current state of knowledge of the functional role of K(+) channels in DSM in health and disease, with special emphasis on current advancements in the field.
Figures

Similar articles
-
Expression and function of K(V)2-containing channels in human urinary bladder smooth muscle.Am J Physiol Cell Physiol. 2012 Jun 1;302(11):C1599-608. doi: 10.1152/ajpcell.00447.2011. Epub 2012 Mar 14. Am J Physiol Cell Physiol. 2012. PMID: 22422395 Free PMC article.
-
Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology.Am J Physiol Regul Integr Comp Physiol. 2014 Sep 15;307(6):R571-84. doi: 10.1152/ajpregu.00142.2014. Epub 2014 Jul 2. Am J Physiol Regul Integr Comp Physiol. 2014. PMID: 24990859 Free PMC article. Review.
-
KV2.1 and electrically silent KV channel subunits control excitability and contractility of guinea pig detrusor smooth muscle.Am J Physiol Cell Physiol. 2012 Jan 15;302(2):C360-72. doi: 10.1152/ajpcell.00303.2010. Epub 2011 Oct 12. Am J Physiol Cell Physiol. 2012. PMID: 21998137 Free PMC article.
-
(-)-(9S)-9-(3-Bromo-4-fluorophenyl)-2,3,5,6,7,9-hexahydrothieno[3,2-b]quinolin-8(4H)-one 1,1-dioxide (A-278637): a novel ATP-sensitive potassium channel opener efficacious in suppressing urinary bladder contractions. I. In vitro characterization.J Pharmacol Exp Ther. 2002 Oct;303(1):379-86. doi: 10.1124/jpet.102.034538. J Pharmacol Exp Ther. 2002. PMID: 12235274
-
Urinary bladder smooth muscle ion channels: expression, function, and regulation in health and disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F257-F283. doi: 10.1152/ajprenal.00048.2020. Epub 2020 Jul 6. Am J Physiol Renal Physiol. 2020. PMID: 32628539 Free PMC article. Review.
Cited by
-
Effect of high-fat diet-induced obesity on the small-conductance Ca2+-activated K+ channel function affecting the contractility of rat detrusor smooth muscle.Int Urol Nephrol. 2019 Jan;51(1):61-72. doi: 10.1007/s11255-018-2016-5. Epub 2018 Oct 25. Int Urol Nephrol. 2019. PMID: 30361965
-
New targets for overactive bladder-ICI-RS 2109.Neurourol Urodyn. 2020 Jul;39 Suppl 3(Suppl 3):S113-S121. doi: 10.1002/nau.24228. Epub 2019 Nov 18. Neurourol Urodyn. 2020. PMID: 31737931 Free PMC article. Review.
-
Properties of single-channel and whole cell Cl- currents in guinea pig detrusor smooth muscle cells.Am J Physiol Cell Physiol. 2019 May 1;316(5):C698-C710. doi: 10.1152/ajpcell.00327.2018. Epub 2018 Dec 19. Am J Physiol Cell Physiol. 2019. PMID: 30566392 Free PMC article.
-
BK channel-mediated relaxation of urinary bladder smooth muscle: a novel paradigm for phosphodiesterase type 4 regulation of bladder function.J Pharmacol Exp Ther. 2014 Apr;349(1):56-65. doi: 10.1124/jpet.113.210708. Epub 2014 Jan 23. J Pharmacol Exp Ther. 2014. PMID: 24459245 Free PMC article.
-
Does hypercholesterolemia affect the relaxation of the detrusor smooth muscle in rats? In vitro and in vivo studies.Naunyn Schmiedebergs Arch Pharmacol. 2015 Jul;388(7):761-71. doi: 10.1007/s00210-014-1060-7. Epub 2014 Oct 26. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25344203
References
-
- Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 2004;84:935–986. - PubMed
-
- Gopalakrishnan M, Shieh CC. Potassium channel subtypes as molecular targets for overactive bladder and other urological disorders. Expert Opin. Ther. Targets. 2004;8:437–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

