The role of ubiquitylation in immune defence and pathogen evasion
- PMID: 22158412
- PMCID: PMC3864900
- DOI: 10.1038/nri3111
The role of ubiquitylation in immune defence and pathogen evasion
Abstract
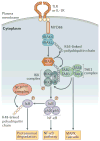
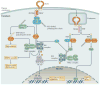
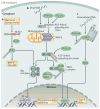
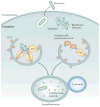
Ubiquitylation is a widely used post-translational protein modification that regulates many biological processes, including immune responses. The role of ubiquitin in immune regulation was originally uncovered through studies of antigen presentation and the nuclear factor-κB family of transcription factors, which orchestrate host defence against microorganisms. Recent studies have revealed crucial roles of ubiquitylation in many aspects of the immune system, including innate and adaptive immunity and antimicrobial autophagy. In addition, mounting evidence indicates that microbial pathogens exploit the ubiquitin pathway to evade the host immune system. Here, we review recent advances on the role of ubiquitylation in host defence and pathogen evasion.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
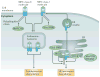
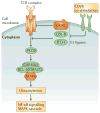
Similar articles
-
The ubiquitin system: a critical regulator of innate immunity and pathogen-host interactions.Cell Mol Immunol. 2016 Sep;13(5):560-76. doi: 10.1038/cmi.2016.40. Epub 2016 Aug 15. Cell Mol Immunol. 2016. PMID: 27524111 Free PMC article. Review.
-
Viral hijacking of the host ubiquitin system to evade interferon responses.Curr Opin Microbiol. 2010 Aug;13(4):517-23. doi: 10.1016/j.mib.2010.05.012. Epub 2010 Jun 17. Curr Opin Microbiol. 2010. PMID: 20699190 Free PMC article. Review.
-
Ubiquitylation in innate and adaptive immunity.Nature. 2009 Mar 26;458(7237):430-7. doi: 10.1038/nature07959. Nature. 2009. PMID: 19325622 Review.
-
Control of MHC II antigen presentation by ubiquitination.Curr Opin Immunol. 2013 Feb;25(1):109-14. doi: 10.1016/j.coi.2012.10.008. Epub 2012 Nov 24. Curr Opin Immunol. 2013. PMID: 23183035 Review.
-
Autophagy in antimicrobial immunity.Mol Cell. 2014 Apr 24;54(2):224-33. doi: 10.1016/j.molcel.2014.03.009. Mol Cell. 2014. PMID: 24766886 Review.
Cited by
-
Mrz1, a Novel Mitochondrial Outer Membrane RING Finger Protein, is Degraded Through the Ubiquitin-Proteasome Pathway in Schizosaccharomyces pombe.Curr Microbiol. 2022 Sep 10;79(10):309. doi: 10.1007/s00284-022-02998-z. Curr Microbiol. 2022. PMID: 36088506
-
YAP promotes the activation of NLRP3 inflammasome via blocking K27-linked polyubiquitination of NLRP3.Nat Commun. 2021 May 11;12(1):2674. doi: 10.1038/s41467-021-22987-3. Nat Commun. 2021. PMID: 33976226 Free PMC article.
-
Integrative Proteomic Analysis of Multiple Posttranslational Modifications in Inflammatory Response.Genomics Proteomics Bioinformatics. 2022 Feb;20(1):163-176. doi: 10.1016/j.gpb.2020.11.004. Epub 2021 Mar 2. Genomics Proteomics Bioinformatics. 2022. PMID: 33662623 Free PMC article.
-
Interplay of m6A and H3K27 trimethylation restrains inflammation during bacterial infection.Sci Adv. 2020 Aug 19;6(34):eaba0647. doi: 10.1126/sciadv.aba0647. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32875102 Free PMC article.
-
The E3 ubiquitin ligase neuregulin receptor degradation protein 1 (Nrdp1) promotes M2 macrophage polarization by ubiquitinating and activating transcription factor CCAAT/enhancer-binding Protein β (C/EBPβ).J Biol Chem. 2012 Aug 3;287(32):26740-8. doi: 10.1074/jbc.M112.383265. Epub 2012 Jun 15. J Biol Chem. 2012. Retraction in: J Biol Chem. 2021 Jan-Jun;296:100755. doi: 10.1016/j.jbc.2021.100755. PMID: 22707723 Free PMC article. Retracted.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

