Enhanced desumoylation in murine hearts by overexpressed SENP2 leads to congenital heart defects and cardiac dysfunction
- PMID: 22155005
- PMCID: PMC3294171
- DOI: 10.1016/j.yjmcc.2011.11.011
Enhanced desumoylation in murine hearts by overexpressed SENP2 leads to congenital heart defects and cardiac dysfunction
Abstract
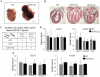
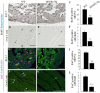
Sumoylation is a posttranslational modification implicated in a variety of cellular activities, and its role in a number of human pathogeneses such as cleft lip/palate has been well documented. However, the importance of the SUMO conjugation pathway in cardiac development and functional disorders is newly emerging. We previously reported that knockout of SUMO-1 in mice led to congenital heart diseases (CHDs). To further investigate the effects of imbalanced SUMO conjugation on heart development and function and its underlying mechanisms, we generated transgenic (Tg) mice with cardiac-specific expression of SENP2, a SUMO-specific protease that deconjugates sumoylated proteins, to evaluate the impact of desumoylation on heart development and function. Overexpression of SENP2 resulted in premature death of mice with CHDs-atrial septal defects (ASDs) and/or ventricular septal defects (VSDs). Immunobiochemistry revealed diminished cardiomyocyte proliferation in SENP2-Tg mouse hearts compared with that in wild type (WT) hearts. Surviving SENP2-Tg mice showed growth retardation, and developed cardiomyopathy with impaired cardiac function with aging. Cardiac-specific overexpression of the SUMO-1 transgene reduced the incidence of cardiac structural phenotypes in the sumoylation defective mice. Moreover, cardiac overexpression of SENP2 in the mice with Nkx2.5 haploinsufficiency promoted embryonic lethality and severity of CHDs, indicating the functional interaction between SENP2 and Nkx2.5 in vivo. Our findings indicate the indispensability of a balanced SUMO pathway for proper cardiac development and function. This article is part of a Special Issue entitled 'Post-translational Modification SI'.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





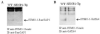

Similar articles
-
Expression of sumoylation deficient Nkx2.5 mutant in Nkx2.5 haploinsufficient mice leads to congenital heart defects.PLoS One. 2011;6(6):e20803. doi: 10.1371/journal.pone.0020803. Epub 2011 Jun 3. PLoS One. 2011. PMID: 21677783 Free PMC article.
-
Inhibition of SENP2-mediated Akt deSUMOylation promotes cardiac regeneration via activating Akt pathway.Clin Sci (Lond). 2021 Mar 26;135(6):811-828. doi: 10.1042/CS20201408. Clin Sci (Lond). 2021. PMID: 33687053
-
Extraembryonic but not embryonic SUMO-specific protease 2 is required for heart development.Sci Rep. 2016 Feb 17;6:20999. doi: 10.1038/srep20999. Sci Rep. 2016. PMID: 26883797 Free PMC article.
-
Cardiac transcription factor Csx/Nkx2-5: Its role in cardiac development and diseases.Pharmacol Ther. 2005 Aug;107(2):252-68. doi: 10.1016/j.pharmthera.2005.03.005. Pharmacol Ther. 2005. PMID: 15925411 Review.
-
SUMO1/sentrin/SMT3 specific peptidase 2 modulates target molecules and its corresponding functions.Biochimie. 2018 Sep;152:6-13. doi: 10.1016/j.biochi.2018.06.007. Epub 2018 Jun 21. Biochimie. 2018. PMID: 29908207 Review.
Cited by
-
Wavelet Screening identifies regions highly enriched for differentially methylated loci for orofacial clefts.NAR Genom Bioinform. 2021 May 3;3(2):lqab035. doi: 10.1093/nargab/lqab035. eCollection 2021 Jun. NAR Genom Bioinform. 2021. PMID: 33987535 Free PMC article.
-
Moderate hypothermia induces protection against hypoxia/reoxygenation injury by enhancing SUMOylation in cardiomyocytes.Mol Med Rep. 2020 Oct;22(4):2617-2626. doi: 10.3892/mmr.2020.11374. Epub 2020 Jul 28. Mol Med Rep. 2020. PMID: 32945433 Free PMC article.
-
DJ-1 protects the heart against ischemia-reperfusion injury by regulating mitochondrial fission.J Mol Cell Cardiol. 2016 Aug;97:56-66. doi: 10.1016/j.yjmcc.2016.04.008. Epub 2016 Apr 22. J Mol Cell Cardiol. 2016. PMID: 27108530 Free PMC article.
-
Developing Practical Therapeutic Strategies that Target Protein SUMOylation.Curr Drug Targets. 2019;20(9):960-969. doi: 10.2174/1389450119666181026151802. Curr Drug Targets. 2019. PMID: 30362419 Free PMC article. Review.
-
Identification of Natural Products as SENP2 Inhibitors for Targeted Therapy in Heart Failure.Front Pharmacol. 2022 Apr 1;13:817990. doi: 10.3389/fphar.2022.817990. eCollection 2022. Front Pharmacol. 2022. PMID: 35431915 Free PMC article.
References
-
- Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106(6):709–21. - PubMed
-
- Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102(10):1169–81. - PubMed
-
- Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126(6):1269–80. - PubMed
-
- Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424(6947):443–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

