Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer
- PMID: 22149875
- PMCID: PMC5705202
- DOI: 10.1056/NEJMoa1113216
Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer
Abstract
Background: The anti-human epidermal growth factor receptor 2 (HER2) humanized monoclonal antibody trastuzumab improves the outcome in patients with HER2-positive metastatic breast cancer. However, most cases of advanced disease eventually progress. Pertuzumab, an anti-HER2 humanized monoclonal antibody that inhibits receptor dimerization, has a mechanism of action that is complementary to that of trastuzumab, and combination therapy with the two antibodies has shown promising activity and an acceptable safety profile in phase 2 studies involving patients with HER2-positive breast cancer.
Methods: We randomly assigned 808 patients with HER2-positive metastatic breast cancer to receive placebo plus trastuzumab plus docetaxel (control group) or pertuzumab plus trastuzumab plus docetaxel (pertuzumab group) as first-line treatment until the time of disease progression or the development of toxic effects that could not be effectively managed. The primary end point was independently assessed progression-free survival. Secondary end points included overall survival, progression-free survival as assessed by the investigator, the objective response rate, and safety.
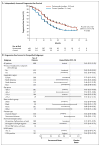
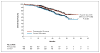
Results: The median progression-free survival was 12.4 months in the control group, as compared with 18.5 months in the pertuzumab group (hazard ratio for progression or death, 0.62; 95% confidence interval, 0.51 to 0.75; P<0.001). The interim analysis of overall survival showed a strong trend in favor of pertuzumab plus trastuzumab plus docetaxel. The safety profile was generally similar in the two groups, with no increase in left ventricular systolic dysfunction; the rates of febrile neutropenia and diarrhea of grade 3 or above were higher in the pertuzumab group than in the control group.
Conclusions: The combination of pertuzumab plus trastuzumab plus docetaxel, as compared with placebo plus trastuzumab plus docetaxel, when used as first-line treatment for HER2-positive metastatic breast cancer, significantly prolonged progression-free survival, with no increase in cardiac toxic effects. (Funded by F. Hoffmann-La Roche/Genentech; ClinicalTrials.gov number, NCT00567190.).
Figures
Comment in
-
HER2 therapy--an abundance of riches.N Engl J Med. 2012 Jan 12;366(2):176-8. doi: 10.1056/NEJMe1113641. Epub 2011 Dec 7. N Engl J Med. 2012. PMID: 22149874 No abstract available.
-
Pertuzumab plus trastuzumab in metastatic breast cancer.N Engl J Med. 2012 Apr 5;366(14):1348; author reply 1349-50. doi: 10.1056/NEJMc1201462. N Engl J Med. 2012. PMID: 22475602 No abstract available.
-
Pertuzumab plus trastuzumab in metastatic breast cancer.N Engl J Med. 2012 Apr 5;366(14):1348-9; author reply 1349-50. doi: 10.1056/NEJMc1201462. N Engl J Med. 2012. PMID: 22475603 No abstract available.
-
Pertuzumab plus trastuzumab in metastatic breast cancer.N Engl J Med. 2012 Apr 5;366(14):1349; author reply 1349-50. doi: 10.1056/NEJMc1201462. N Engl J Med. 2012. PMID: 22475604 No abstract available.
Similar articles
-
Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study.Lancet Oncol. 2013 May;14(6):461-71. doi: 10.1016/S1470-2045(13)70130-X. Epub 2013 Apr 18. Lancet Oncol. 2013. PMID: 23602601 Free PMC article. Clinical Trial.
-
Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer.N Engl J Med. 2015 Feb 19;372(8):724-34. doi: 10.1056/NEJMoa1413513. N Engl J Med. 2015. PMID: 25693012 Free PMC article. Clinical Trial.
-
Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study.Lancet Oncol. 2020 Apr;21(4):519-530. doi: 10.1016/S1470-2045(19)30863-0. Epub 2020 Mar 12. Lancet Oncol. 2020. PMID: 32171426 Clinical Trial.
-
Pertuzumab: a review of its use for first-line combination treatment of HER2-positive metastatic breast cancer.Drugs. 2013 Sep;73(13):1491-502. doi: 10.1007/s40265-013-0109-0. Drugs. 2013. PMID: 23982598 Review.
-
Pertuzumab in combination with trastuzumab and docetaxel for HER2-positive metastatic breast cancer.Expert Rev Anticancer Ther. 2015 Jan;15(1):17-26. doi: 10.1586/14737140.2015.992418. Epub 2014 Dec 12. Expert Rev Anticancer Ther. 2015. PMID: 25494663 Review.
Cited by
-
Trastuzumab, Pertuzumab, and Docetaxel as the First Line for HER-2-Positive Metastatic Breast Cancer among Arabs.Breast Care (Basel). 2021 Feb;16(1):59-65. doi: 10.1159/000506824. Epub 2020 Apr 6. Breast Care (Basel). 2021. PMID: 33716633 Free PMC article.
-
The Influence of Pre-Existing Beta-Blockers Use on Survival Outcomes in HER2 Positive Advanced Breast Cancer: Pooled Analysis of Clinical Trial Data.Front Oncol. 2020 Jul 14;10:1130. doi: 10.3389/fonc.2020.01130. eCollection 2020. Front Oncol. 2020. PMID: 32760671 Free PMC article.
-
Targeted Therapies in Older Adults With Solid Tumors.J Clin Oncol. 2021 Jul 1;39(19):2128-2137. doi: 10.1200/JCO.21.00132. Epub 2021 May 27. J Clin Oncol. 2021. PMID: 34043448 Free PMC article. Review. No abstract available.
-
Inhibition of triple-negative breast cancer models by combinations of antibodies to EGFR.Proc Natl Acad Sci U S A. 2013 Jan 29;110(5):1815-20. doi: 10.1073/pnas.1220763110. Epub 2013 Jan 14. Proc Natl Acad Sci U S A. 2013. PMID: 23319610 Free PMC article.
-
The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy.Chemother Res Pract. 2012;2012:743193. doi: 10.1155/2012/743193. Epub 2012 Dec 20. Chemother Res Pract. 2012. PMID: 23320171 Free PMC article.
References
-
- Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. - PubMed
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a mono-clonal antibody against HER2 for meta-static breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. - PubMed
-
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74. - PubMed
-
- Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
