Macromolecular crowding regulates assembly of mRNA stress granules after osmotic stress: new role for compatible osmolytes
- PMID: 22147700
- PMCID: PMC3268405
- DOI: 10.1074/jbc.M111.292748
Macromolecular crowding regulates assembly of mRNA stress granules after osmotic stress: new role for compatible osmolytes
Abstract
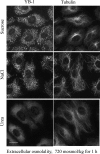
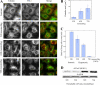
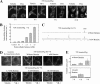
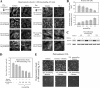
The massive uptake of compatible osmolytes such as betaine, taurine, and myo-inositol is a protective response shared by all eukaryotes exposed to hypertonic stress. Their accumulation results mostly from the expression of specific transporters triggered by the transcriptional factor NFAT5/TonEBP. This allows the recovery of the cell volume without increasing intracellular ionic strength. In this study we consider the assembly and dissociation of mRNA stress granules (SGs) in hypertonic-stressed cells and the role of compatible osmolytes. In agreement with in vitro results obtained on isolated mRNAs, both macromolecular crowding and a high ionic strength favor the assembly of SGs in normal rat kidney epithelial cells. However, after hours of constant hypertonicity, the slow accumulation in the cytoplasm of compatible osmolytes via specific transporters both reduces macromolecular crowding and ionic strength, thus leading to the progressive dissociation of SGs. In line with this, when cells are exposed to hypertonicity to accumulate a large amount of compatible osmolytes, the formation of SGs is severely impaired, and cells increase their chances of survival to another hypertonic episode. Altogether, these results indicate that the impact of compatible osmolytes on the mRNA-associated machineries and especially that associated with SGs may play an important role in cell resistance and adaption to hyperosmolarity in many tissues like kidney and liver.
Figures



Similar articles
-
Compatible organic osmolytes in rat liver sinusoidal endothelial cells.Hepatology. 1998 Feb;27(2):569-75. doi: 10.1002/hep.510270235. Hepatology. 1998. PMID: 9462659
-
Gap junctions favor normal rat kidney epithelial cell adaptation to chronic hypertonicity.Am J Physiol Cell Physiol. 2011 Sep;301(3):C705-16. doi: 10.1152/ajpcell.00128.2011. Epub 2011 Jun 15. Am J Physiol Cell Physiol. 2011. PMID: 21677260
-
Compatible osmolytes modulate the response of porcine endothelial cells to hypertonicity and protect them from apoptosis.J Physiol. 2002 Apr 15;540(Pt 2):499-508. doi: 10.1113/jphysiol.2001.013395. J Physiol. 2002. PMID: 11956339 Free PMC article.
-
Molecular basis of osmotic regulation.Am J Physiol. 1995 Jun;268(6 Pt 2):F983-96. doi: 10.1152/ajprenal.1995.268.6.F983. Am J Physiol. 1995. PMID: 7611465 Review.
-
Kidney cell survival in high tonicity.Comp Biochem Physiol A Physiol. 1997 Jul;117(3):301-6. doi: 10.1016/s0300-9629(96)00267-8. Comp Biochem Physiol A Physiol. 1997. PMID: 9172386 Review.
Cited by
-
Increased TGF-β and BMP Levels and Improved Chondrocyte-Specific Marker Expression In Vitro under Cartilage-Specific Physiological Osmolarity.Int J Mol Sci. 2019 Feb 13;20(4):795. doi: 10.3390/ijms20040795. Int J Mol Sci. 2019. PMID: 30781744 Free PMC article.
-
RNA self-assembly contributes to stress granule formation and defining the stress granule transcriptome.Proc Natl Acad Sci U S A. 2018 Mar 13;115(11):2734-2739. doi: 10.1073/pnas.1800038115. Epub 2018 Feb 26. Proc Natl Acad Sci U S A. 2018. PMID: 29483269 Free PMC article.
-
A little less aggregation a little more replication: Viral manipulation of stress granules.Wiley Interdiscip Rev RNA. 2023 Jan;14(1):e1741. doi: 10.1002/wrna.1741. Epub 2022 Jun 16. Wiley Interdiscip Rev RNA. 2023. PMID: 35709333 Free PMC article. Review.
-
Macromolecular Crowding Enhances Catalytic Efficiency and Stability of α-Amylase.ISRN Biotechnol. 2012 Nov 8;2013:737805. doi: 10.5402/2013/737805. eCollection 2013. ISRN Biotechnol. 2012. PMID: 25969780 Free PMC article.
-
Taurine suppresses liquid-liquid phase separation of lysozyme protein.Amino Acids. 2021 May;53(5):745-751. doi: 10.1007/s00726-021-02980-2. Epub 2021 Apr 21. Amino Acids. 2021. PMID: 33881613
References
-
- Strange K. (2004) Cellular volume homeostasis. Adv. Physiol. Educ 28, 155–159 - PubMed
-
- Wehner F., Olsen H., Tinel H., Kinne-Saffran E., Kinne R. K. (2003) Cell volume regulation. Osmolytes, osmolyte transport, and signal transduction. Rev. Physiol. Biochem. Pharmacol 148, 1–80 - PubMed
-
- Grinstein S., Woodside M., Sardet C., Pouyssegur J., Rotin D. (1992) Activation of the Na+/H+ antiporter during cell volume regulation. Evidence for a phosphorylation-independent mechanism. J. Biol. Chem. 267, 23823–23828 - PubMed
-
- Strange K., Emma F., Jackson P. S. (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am. J. Physiol. 270, C711–C730 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

