Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice
- PMID: 22147014
- PMCID: PMC3275394
- DOI: 10.1210/en.2011-1599
Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice
Abstract
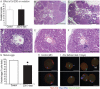
Shortly before ovulation, the oocyte acquires developmental competence and granulosa cells undergo tremendous changes including cumulus expansion and luteinization. Zinc is emerging as a key regulator of meiosis in vitro, but a complete understanding of zinc-mediated effects during the periovulatory period is lacking. The present study uncovers the previously unknown role of zinc in maintaining meiotic arrest before ovulation. A zinc chelator [N,N,N',N'-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN)] caused premature germinal vesicle breakdown and associated spindle defects in denuded oocytes even in the presence of a phosphodiesterase 3A inhibitor (milrinone). TPEN also potently blocked cumulus expansion by blocking induction of expansion-related transcripts Has2, Ptx3, Ptgs2, and Tnfaip6 mRNA. Both meiotic arrest and cumulus expansion were rescued by exogenous zinc. Lack of cumulus expansion is due to an almost complete suppression of phospho-Sma- and Mad-related protein 2/3 signaling. Consistent with a decrease in phospho-Sma- and Mad-related protein 2/3 signaling, TPEN also decreased cumulus transcripts (Ar and Slc38a3) and caused a surprising increase in mural transcripts (Lhcgr and Cyp11a1) in cumulus cells. In vivo, feeding a zinc-deficient diet for 10 d completely blocked ovulation and compromised cumulus expansion. However, 42.5% of oocytes had prematurely resumed meiosis before human chorionic gonadotropin injection, underscoring the importance of zinc before ovulation. A more acute 3-d treatment with a zinc-deficient diet did not block ovulation but did increase the number of oocytes trapped in luteinizing follicles. Moreover, 23% of ovulated oocytes did not reach metaphase II due to severe spindle defects. Thus, acute zinc deficiency causes profound defects during the periovulatory period with consequences for oocyte maturation, cumulus expansion, and ovulation.
Figures
Similar articles
-
Transition Metal Chelator Induces Progesterone Production in Mouse Cumulus-Oocyte Complexes and Corpora Lutea.Biol Trace Elem Res. 2017 Apr;176(2):374-383. doi: 10.1007/s12011-016-0841-x. Epub 2016 Sep 7. Biol Trace Elem Res. 2017. PMID: 27604975
-
Signaling pathways regulating FSH- and amphiregulin-induced meiotic resumption and cumulus cell expansion in the pig.Reproduction. 2012 Nov;144(5):535-46. doi: 10.1530/REP-12-0191. Epub 2012 Sep 4. Reproduction. 2012. PMID: 22949725
-
The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion.Dev Biol. 2006 Nov 1;299(1):91-104. doi: 10.1016/j.ydbio.2006.07.012. Epub 2006 Jul 15. Dev Biol. 2006. PMID: 16908014
-
The epidermal growth factor network: role in oocyte growth, maturation and developmental competence.Hum Reprod Update. 2018 Jan 1;24(1):1-14. doi: 10.1093/humupd/dmx029. Hum Reprod Update. 2018. PMID: 29029246 Review.
-
Roles of epidermal growth factor (EGF)-like factor in the ovulation process.Reprod Med Biol. 2016 Feb 15;15(4):201-216. doi: 10.1007/s12522-016-0236-x. eCollection 2016 Oct. Reprod Med Biol. 2016. PMID: 29259438 Free PMC article. Review.
Cited by
-
Role of zinc in female reproduction.Biol Reprod. 2021 May 7;104(5):976-994. doi: 10.1093/biolre/ioab023. Biol Reprod. 2021. PMID: 33598687 Free PMC article. Review.
-
Follicle-intrinsic and spatially distinct molecular programs drive follicle rupture and luteinization during ex vivo mammalian ovulation.Commun Biol. 2024 Oct 23;7(1):1374. doi: 10.1038/s42003-024-07074-9. Commun Biol. 2024. PMID: 39443665 Free PMC article.
-
Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS-MAPK pathway.Biol Reprod. 2012 Jul 1;87(1):11, 1-12. doi: 10.1095/biolreprod.112.099390. Print 2012 Jul. Biol Reprod. 2012. PMID: 22539682 Free PMC article.
-
Zinc's Association with the CmPn/CmP Signaling Network in Breast Cancer Tumorigenesis.Biomolecules. 2022 Nov 11;12(11):1672. doi: 10.3390/biom12111672. Biomolecules. 2022. PMID: 36421686 Free PMC article. Review.
-
The zinc spark is an inorganic signature of human egg activation.Sci Rep. 2016 Apr 26;6:24737. doi: 10.1038/srep24737. Sci Rep. 2016. PMID: 27113677 Free PMC article.
References
-
- Mehlmann LM, Saeki Y, Tanaka S, Brennan TJ, Evsikov AV, Pendola FL, Knowles BB, Eppig JJ, Jaffe LA. 2004. The Gs-linked receptor GPR3 maintains meiotic arrest in mammalian oocytes. Science 306:1947–1950 - PubMed
-
- Hinckley M, Vaccari S, Horner K, Chen R, Conti M. 2005. The G-protein-coupled receptors GPR3 and GPR12 are involved in cAMP signaling and maintenance of meiotic arrest in rodent oocytes. Dev Biol 287:249–261 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

