Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant
- PMID: 22143789
- PMCID: PMC3251097
- DOI: 10.1073/pnas.1108360108
Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant
Abstract
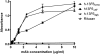
No countermeasures currently exist for the prevention or treatment of the severe sequelae of Filovirus (such as Ebola virus; EBOV) infection. To overcome this limitation in our biodefense preparedness, we have designed monoclonal antibodies (mAbs) which could be used in humans as immunoprotectants for EBOV, starting with a murine mAb (13F6) that recognizes the heavily glycosylated mucin-like domain of the virion-attached glycoprotein (GP). Point mutations were introduced into the variable region of the murine mAb to remove predicted human T-cell epitopes, and the variable regions joined to human constant regions to generate a mAb (h-13F6) appropriate for development for human use. We have evaluated the efficacy of three variants of h-13F6 carrying different glycosylation patterns in a lethal mouse EBOV challenge model. The pattern of glycosylation of the various mAbs was found to correlate to level of protection, with aglycosylated h-13F6 providing the least potent efficacy (ED(50) = 33 μg). A version with typical heterogenous mammalian glycoforms (ED(50) = 11 μg) had similar potency to the original murine mAb. However, h-13F6 carrying complex N-glycosylation lacking core fucose exhibited superior potency (ED(50) = 3 μg). Binding studies using Fcγ receptors revealed enhanced binding of nonfucosylated h-13F6 to mouse and human FcγRIII. Together the results indicate the presence of Fc N-glycans enhances the protective efficacy of h-13F6, and that mAbs manufactured with uniform glycosylation and a higher potency glycoform offer promise as biodefense therapeutics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Expression of a Large Single-Chain 13F6 Antibody with Binding Activity against Ebola Virus-Like Particles in a Plant System.Int J Mol Sci. 2020 Sep 23;21(19):7007. doi: 10.3390/ijms21197007. Int J Mol Sci. 2020. PMID: 32977599 Free PMC article.
-
Tobacco against Ebola virus disease.Przegl Lek. 2015;72(10):567-71. Przegl Lek. 2015. PMID: 26946569 Review.
-
Pan-ebolavirus and Pan-filovirus Mouse Monoclonal Antibodies: Protection against Ebola and Sudan Viruses.J Virol. 2015 Oct 14;90(1):266-78. doi: 10.1128/JVI.02171-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468533 Free PMC article.
-
Macaque Monoclonal Antibodies Targeting Novel Conserved Epitopes within Filovirus Glycoprotein.J Virol. 2015 Oct 14;90(1):279-91. doi: 10.1128/JVI.02172-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26468532 Free PMC article.
-
The emergence of antibody therapies for Ebola.Hum Antibodies. 2015 Dec 23;23(3-4):49-56. doi: 10.3233/HAB-150284. Hum Antibodies. 2015. PMID: 27472862 Review.
Cited by
-
Discovery and early development of AVI-7537 and AVI-7288 for the treatment of Ebola virus and Marburg virus infections.Viruses. 2012 Nov 6;4(11):2806-30. doi: 10.3390/v4112806. Viruses. 2012. PMID: 23202506 Free PMC article.
-
Monoclonal antibody therapy for Junin virus infection.Proc Natl Acad Sci U S A. 2016 Apr 19;113(16):4458-63. doi: 10.1073/pnas.1600996113. Epub 2016 Apr 4. Proc Natl Acad Sci U S A. 2016. PMID: 27044104 Free PMC article.
-
Site-Selective Chemoenzymatic Modification on the Core Fucose of an Antibody Enhances Its Fcγ Receptor Affinity and ADCC Activity.J Am Chem Soc. 2021 May 26;143(20):7828-7838. doi: 10.1021/jacs.1c03174. Epub 2021 May 12. J Am Chem Soc. 2021. PMID: 33977722 Free PMC article.
-
The impact of N-glycans on the immune response of plant-produced SARS-CoV-2 RBD-Fc proteins.Biotechnol Rep (Amst). 2024 Jun 20;43:e00847. doi: 10.1016/j.btre.2024.e00847. eCollection 2024 Sep. Biotechnol Rep (Amst). 2024. PMID: 39040987 Free PMC article.
-
Omicron variant Spike-specific antibody binding and Fc activity are preserved in recipients of mRNA or inactivated COVID-19 vaccines.Sci Transl Med. 2022 Apr 27;14(642):eabn9243. doi: 10.1126/scitranslmed.abn9243. Epub 2022 Apr 27. Sci Transl Med. 2022. PMID: 35289637 Free PMC article.
References
-
- Alibek K. Biohazard: The Chilling True Story of the Largest Covert Biological Weapons Program in the World–Told from Inside by the Man Who Ran It. New York: Random House; 1999.
-
- Wilson JA, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. - PubMed
-
- Jones TD, Crompton LJ, Carr FJ, Baker MP. Deimmunization of monoclonal antibodies. Methods Mol Biol. 2009;525:405–423, xiv. - PubMed
-
- Jefferis R. Glycosylation of recombinant antibody therapeutics. Biotechnol Prog. 2005;21:11–16. - PubMed
-
- Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8:226–234. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

