Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity
- PMID: 22138715
- PMCID: PMC3242938
- DOI: 10.1038/ni.2182
Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity
Abstract
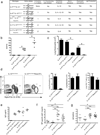
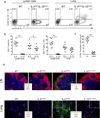
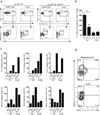
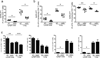
Interleukin 4 (IL-4) and IL-13 are critical for responses to parasitic helminthes. We used genetically engineered reporter mice to assess the temporal and spatial production of these cytokines in vivo. In lymph nodes, IL-4, but not IL-13, was made by follicular helper T cells (T(FH) cells). In contrast, tissue type 2 helper T cells (T(H)2 cells) produced both cytokines. There was also divergent production of IL-4 and IL-13 among cells of the innate immune system, whereby basophils produced IL-4, whereas innate helper type 2 cells (Ih2 cells) produced IL-13. IL-13 production by T(H)2 and Ih2 cells was dependent on the transcription factor GATA-3, which was present in large amounts in these cells, and in contrast to the small amount of GATA-3 in T(FH) cells and basophils. The distinct localization and cellular expression of IL-4 and IL-13 explains their unique roles during allergic immunity.
Figures




Similar articles
-
T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production.Cytokine. 2015 Sep;75(1):14-24. doi: 10.1016/j.cyto.2015.05.010. Epub 2015 Jun 1. Cytokine. 2015. PMID: 26044597 Free PMC article. Review.
-
IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils.Proc Natl Acad Sci U S A. 1999 Nov 23;96(24):13962-6. doi: 10.1073/pnas.96.24.13962. Proc Natl Acad Sci U S A. 1999. PMID: 10570181 Free PMC article.
-
Basophils are the major producers of IL-4 during primary helminth infection.J Immunol. 2011 Mar 1;186(5):2719-28. doi: 10.4049/jimmunol.1000940. Epub 2011 Jan 26. J Immunol. 2011. PMID: 21270410 Free PMC article.
-
Regulation of Th2 cytokine expression in NKT cells: unconventional use of Stat6, GATA-3, and NFAT2.J Immunol. 2006 Jan 15;176(2):880-8. doi: 10.4049/jimmunol.176.2.880. J Immunol. 2006. PMID: 16393972
-
Basophils: emerging roles in the pathogenesis of allergic disease.Immunol Rev. 2011 Jul;242(1):144-60. doi: 10.1111/j.1600-065X.2011.01023.x. Immunol Rev. 2011. PMID: 21682743 Review.
Cited by
-
The peptide specificity of the endogenous T follicular helper cell repertoire generated after protein immunization.PLoS One. 2012;7(10):e46952. doi: 10.1371/journal.pone.0046952. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077537 Free PMC article.
-
Distinct spatial and temporal roles for Th1, Th2, and Th17 cells in asthma.Front Immunol. 2022 Aug 12;13:974066. doi: 10.3389/fimmu.2022.974066. eCollection 2022. Front Immunol. 2022. PMID: 36032162 Free PMC article. Review.
-
Murine parainfluenza virus persists in lung innate immune cells sustaining chronic lung pathology.Nat Microbiol. 2024 Nov;9(11):2803-2816. doi: 10.1038/s41564-024-01805-8. Epub 2024 Oct 2. Nat Microbiol. 2024. PMID: 39358466
-
Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues.Nat Immunol. 2013 Mar;14(3):221-9. doi: 10.1038/ni.2534. Epub 2013 Jan 20. Nat Immunol. 2013. PMID: 23334791
-
T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production.Cytokine. 2015 Sep;75(1):14-24. doi: 10.1016/j.cyto.2015.05.010. Epub 2015 Jun 1. Cytokine. 2015. PMID: 26044597 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

