Host-pathogen interactions between the skin and Staphylococcus aureus
- PMID: 22137885
- PMCID: PMC3265682
- DOI: 10.1016/j.mib.2011.11.003
Host-pathogen interactions between the skin and Staphylococcus aureus
Abstract
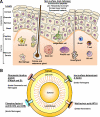
Staphylococcus aureus is responsible for the vast majority of bacterial skin infections in humans. The propensity for S. aureus to infect skin involves a balance between cutaneous immune defense mechanisms and virulence factors of the pathogen. The tissue architecture of the skin is different from other epithelia especially since it possesses a corneal layer, which is an important barrier that protects against the pathogenic microorganisms in the environment. The skin surface, epidermis, and dermis all contribute to host defense against S. aureus. Conversely, S. aureus utilizes various mechanisms to evade these host defenses to promote colonization and infection of the skin. This review will focus on host-pathogen interactions at the skin interface during the pathogenesis of S. aureus colonization and infection.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
The immunopathogenesis of staphylococcal skin infections - A review.Comp Immunol Microbiol Infect Dis. 2016 Dec;49:8-28. doi: 10.1016/j.cimid.2016.08.004. Epub 2016 Aug 13. Comp Immunol Microbiol Infect Dis. 2016. PMID: 27865269 Review.
-
Colonization and infection of the skin by S. aureus: immune system evasion and the response to cationic antimicrobial peptides.Int J Mol Sci. 2014 May 16;15(5):8753-72. doi: 10.3390/ijms15058753. Int J Mol Sci. 2014. PMID: 24840573 Free PMC article. Review.
-
Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease.Trends Microbiol. 2019 Jun;27(6):497-507. doi: 10.1016/j.tim.2019.01.008. Epub 2019 Mar 5. Trends Microbiol. 2019. PMID: 30846311 Free PMC article. Review.
-
Cutaneous bacteria induce immunosuppression.Oncotarget. 2015 Oct 13;6(31):30441-2. doi: 10.18632/oncotarget.5962. Oncotarget. 2015. PMID: 26450905 Free PMC article. No abstract available.
-
Staphylococcus aureus Fatty Acid Kinase FakA Modulates Pathogenesis during Skin Infection via Proteases.Infect Immun. 2020 Jul 21;88(8):e00163-20. doi: 10.1128/IAI.00163-20. Print 2020 Jul 21. Infect Immun. 2020. PMID: 32513856 Free PMC article.
Cited by
-
WYBQ-4: a New Bactericidal Agent against Methicillin-Resistant Staphylococcus aureus.Microbiol Spectr. 2022 Oct 26;10(5):e0054722. doi: 10.1128/spectrum.00547-22. Epub 2022 Sep 13. Microbiol Spectr. 2022. PMID: 36098533 Free PMC article.
-
Antimicrobial Peptides and Their Therapeutic Potential for Bacterial Skin Infections and Wounds.Front Pharmacol. 2018 Mar 28;9:281. doi: 10.3389/fphar.2018.00281. eCollection 2018. Front Pharmacol. 2018. PMID: 29643807 Free PMC article. Review.
-
Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance.PLoS Pathog. 2014 Jun 12;10(6):e1004174. doi: 10.1371/journal.ppat.1004174. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945495 Free PMC article.
-
Community-Associated Methicillin-Resistant Staphylococcus aureus: An Enemy amidst Us.PLoS Pathog. 2016 Oct 6;12(10):e1005837. doi: 10.1371/journal.ppat.1005837. eCollection 2016 Oct. PLoS Pathog. 2016. PMID: 27711163 Free PMC article. Review. No abstract available.
-
Three Lipid Emulsions Reduce Staphylococcus aureus-Stimulated Phagocytosis in Mouse RAW264.7 Cells.Microorganisms. 2021 Nov 30;9(12):2479. doi: 10.3390/microorganisms9122479. Microorganisms. 2021. PMID: 34946079 Free PMC article.
References
-
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N.Engl.J.Med. 2006;355:666–674. - PubMed
-
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N.Engl.J.Med. 2007;357:380–390. - PubMed
-
- Elston DM. Community-acquired methicillin-resistant Staphylococcus aureus. J.Am.Acad.Dermatol. 2007;56:1–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

