A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory
- PMID: 22118528
- PMCID: PMC3228524
- DOI: 10.1016/j.immuni.2011.09.016
A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory
Abstract
STAT3 transcription factor signaling in specific T helper cell differentiation has been well described, although the broader roles for STAT3 in lymphocyte memory are less clear. Patients with autosomal-dominant hyper-IgE syndrome (AD-HIES) carry dominant-negative STAT3 mutations and are susceptible to a variety of bacterial and fungal infections. We found that AD-HIES patients have a cell-intrinsic defect in the number of central memory CD4(+) and CD8(+) T cells compared to healthy controls. Naive T cells from AD-HIES patients had lower expression of memory-related transcription factors BCL6 and SOCS3, a primary proliferation defect, and they failed to acquire central memory-like surface phenotypes in vitro. AD-HIES patients showed a decreased ability to control varicella zoster virus (VZV) and Epstein-Barr virus (EBV) latency, and T cell memory to both of these viruses was compromised. These data point to a specific role for STAT3 in human central memory T cell formation and in control of certain chronic viruses.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
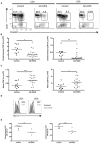
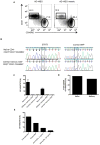

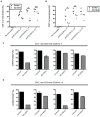
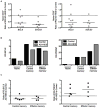
Comment in
-
Keeping STATs on memory CD8+ T cells.Immunity. 2011 Nov 23;35(5):663-5. doi: 10.1016/j.immuni.2011.11.006. Immunity. 2011. PMID: 22118521
Similar articles
-
Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function.J Allergy Clin Immunol. 2013 Aug;132(2):400-11.e9. doi: 10.1016/j.jaci.2013.05.029. Epub 2013 Jul 4. J Allergy Clin Immunol. 2013. PMID: 23830147 Free PMC article.
-
Impaired memory B-cell development and antibody maturation with a skewing toward IgE in patients with STAT3 hyper-IgE syndrome.Allergy. 2019 Dec;74(12):2394-2405. doi: 10.1111/all.13969. Epub 2019 Aug 16. Allergy. 2019. PMID: 31269238
-
Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation.J Allergy Clin Immunol. 2013 Dec;132(6):1388-96. doi: 10.1016/j.jaci.2013.08.045. Epub 2013 Nov 1. J Allergy Clin Immunol. 2013. PMID: 24184145 Free PMC article.
-
Clinical Manifestations and Genetic Analysis of 17 Patients with Autosomal Dominant Hyper-IgE Syndrome in Mainland China: New Reports and a Literature Review.J Clin Immunol. 2017 Feb;37(2):166-179. doi: 10.1007/s10875-017-0369-7. Epub 2017 Feb 14. J Clin Immunol. 2017. PMID: 28197791 Review.
-
Hyperimmunoglobulin E syndromes in pediatrics.Curr Opin Pediatr. 2011 Dec;23(6):653-8. doi: 10.1097/MOP.0b013e32834c7f65. Curr Opin Pediatr. 2011. PMID: 21970826 Free PMC article. Review.
Cited by
-
An Inhibitory Role for the Transcription Factor Stat3 in Controlling IL-4 and Bcl6 Expression in Follicular Helper T Cells.J Immunol. 2015 Sep 1;195(5):2080-9. doi: 10.4049/jimmunol.1500335. Epub 2015 Jul 17. J Immunol. 2015. PMID: 26188063 Free PMC article.
-
B-lymphocyte lineage cells and the respiratory system.J Allergy Clin Immunol. 2013 Apr;131(4):933-57; quiz 958. doi: 10.1016/j.jaci.2013.02.023. J Allergy Clin Immunol. 2013. PMID: 23540615 Free PMC article. Review.
-
Disparate roles for STAT5 in primary and secondary CTL responses.J Immunol. 2013 Apr 1;190(7):3390-8. doi: 10.4049/jimmunol.1202674. Epub 2013 Feb 25. J Immunol. 2013. PMID: 23440411 Free PMC article.
-
DIALing-up the preclinical characterization of gene-modified adoptive cellular immunotherapies.Front Immunol. 2023 Nov 28;14:1264882. doi: 10.3389/fimmu.2023.1264882. eCollection 2023. Front Immunol. 2023. PMID: 38090585 Free PMC article. Review.
-
Intersection of mTOR and STAT signaling in immunity.Trends Immunol. 2015 Jan;36(1):21-9. doi: 10.1016/j.it.2014.10.006. Epub 2014 Nov 15. Trends Immunol. 2015. PMID: 25592035 Free PMC article. Review.
References
-
- Akaishi H, Takeda K, Kaisho T, Shineha R, Satomi S, Takeda J, Akira S. Defective IL-2-mediated IL-2 receptor alpha chain expression in Stat3-deficient T lymphocytes. Int Immunol. 1998;10:1747–1751. - PubMed
-
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. - PubMed
-
- Buckley RH, Schiff SE, Hayward AR. Reduced frequency of CD45RO+ T lymphocytes in blood of hyper-IgE syndrome patients. J Allergy Clin Immunol. 1991;87:313.
-
- Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

