Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs
- PMID: 22118459
- PMCID: PMC3255795
- DOI: 10.1016/j.cell.2011.09.055
Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs
Abstract
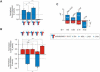
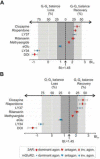
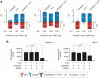
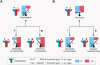
Atypical antipsychotic drugs, such as clozapine and risperidone, have a high affinity for the serotonin 5-HT(2A) G protein-coupled receptor (GPCR), the 2AR, which signals via a G(q) heterotrimeric G protein. The closely related non-antipsychotic drugs, such as ritanserin and methysergide, also block 2AR function, but they lack comparable neuropsychological effects. Why some but not all 2AR inhibitors exhibit antipsychotic properties remains unresolved. We now show that a heteromeric complex between the 2AR and the G(i)-linked GPCR, metabotropic glutamate 2 receptor (mGluR2), integrates ligand input, modulating signaling output and behavioral changes. Serotonergic and glutamatergic drugs bind the mGluR2/2AR heterocomplex, which then balances Gi- and Gq-dependent signaling. We find that the mGluR2/2AR-mediated changes in Gi and Gq activity predict the psychoactive behavioral effects of a variety of pharmocological compounds. These observations provide mechanistic insight into antipsychotic action that may advance therapeutic strategies for disorders including schizophrenia and dementia.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Anti-/Propsychotic drug signaling via heteromeric GPCRs--a balancing act?Cell. 2011 Nov 23;147(5):964-5. doi: 10.1016/j.cell.2011.11.012. Cell. 2011. PMID: 22118451
Similar articles
-
Cross-signaling in metabotropic glutamate 2 and serotonin 2A receptor heteromers in mammalian cells.Pflugers Arch. 2016 May;468(5):775-93. doi: 10.1007/s00424-015-1780-7. Epub 2016 Jan 16. Pflugers Arch. 2016. PMID: 26780666 Free PMC article.
-
Chronic clozapine treatment restrains via HDAC2 the performance of mGlu2 receptor agonism in a rodent model of antipsychotic activity.Neuropsychopharmacology. 2019 Jan;44(2):443-454. doi: 10.1038/s41386-018-0143-4. Epub 2018 Jul 7. Neuropsychopharmacology. 2019. PMID: 30038413 Free PMC article.
-
The crosstalk between 5-HT2AR and mGluR2 in schizophrenia.Neuropharmacology. 2023 Jun 1;230:109489. doi: 10.1016/j.neuropharm.2023.109489. Epub 2023 Mar 6. Neuropharmacology. 2023. PMID: 36889432 Free PMC article. Review.
-
Inhibition by various antipsychotic drugs of the G-protein-activated inwardly rectifying K(+) (GIRK) channels expressed in xenopus oocytes.Br J Pharmacol. 2000 Apr;129(8):1716-22. doi: 10.1038/sj.bjp.0703224. Br J Pharmacol. 2000. PMID: 10780978 Free PMC article.
-
Role of interaction of mGlu2 and 5-HT2A receptors in antipsychotic effects.Pharmacol Biochem Behav. 2022 Nov;221:173474. doi: 10.1016/j.pbb.2022.173474. Epub 2022 Oct 14. Pharmacol Biochem Behav. 2022. PMID: 36244526 Review.
Cited by
-
Repressive epigenetic changes at the mGlu2 promoter in frontal cortex of 5-HT2A knockout mice.Mol Pharmacol. 2013 Jun;83(6):1166-75. doi: 10.1124/mol.112.084582. Epub 2013 Mar 18. Mol Pharmacol. 2013. PMID: 23508685 Free PMC article.
-
Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: Still promising or a dead end?Prog Neuropsychopharmacol Biol Psychiatry. 2015 Jul 3;60:66-76. doi: 10.1016/j.pnpbp.2015.02.012. Epub 2015 Feb 24. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25724760 Free PMC article. Review.
-
The prevalence, maintenance, and relevance of G protein-coupled receptor oligomerization.Mol Pharmacol. 2013 Jul;84(1):158-69. doi: 10.1124/mol.113.084780. Epub 2013 Apr 30. Mol Pharmacol. 2013. PMID: 23632086 Free PMC article. Review.
-
Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: the missing link?Front Pharmacol. 2015 Mar 17;6:46. doi: 10.3389/fphar.2015.00046. eCollection 2015. Front Pharmacol. 2015. PMID: 25852551 Free PMC article. Review.
-
Developmental decline in modulation of glutamatergic synapses in layer IV of the barrel cortex by group II metabotropic glutamate receptors.Neuroscience. 2015 Apr 2;290:41-8. doi: 10.1016/j.neuroscience.2014.12.083. Epub 2015 Jan 13. Neuroscience. 2015. PMID: 25595969 Free PMC article.
References
-
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol. Ther. 2009;121:160–173. - PubMed
-
- Bardenstein LM, Gurovich IY, Morozova MA, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 2007;13:1102–1107. - PubMed
-
- Barducci A, Bussi G, Parrinello M. Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys. Rev. Lett. 2008;100:020603. - PubMed
-
- Bespalov A, Jongen-Relo AL, van Gaalen M, Harich S, Schoemaker H, Gross G. Habituation deficits induced by metabotropic glutamate receptors 2/3 receptor blockade in mice: reversal by antipsychotic drugs. J. Pharmacol. Exp. Ther. 2007;320:944–950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 MH091360/MH/NIMH NIH HHS/United States
- F30 HL097582/HL/NHLBI NIH HHS/United States
- F30HL097582/HL/NHLBI NIH HHS/United States
- MH084894/MH/NIMH NIH HHS/United States
- K02 DA026434/DA/NIDA NIH HHS/United States
- R01 MH084894/MH/NIMH NIH HHS/United States
- SRC1DA02811202/DA/NIDA NIH HHS/United States
- 1R01DA02694702/DA/NIDA NIH HHS/United States
- 5R01MH084894/MH/NIMH NIH HHS/United States
- MH091360/MH/NIMH NIH HHS/United States
- R01 HL059949/HL/NHLBI NIH HHS/United States
- DA026434/DA/NIDA NIH HHS/United States
- R56 MH084894/MH/NIMH NIH HHS/United States
- HL59949/HL/NHLBI NIH HHS/United States
- R01 HL059949-15/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

