Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II
- PMID: 22117068
- PMCID: PMC3256896
- DOI: 10.1074/jbc.M111.294959
Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II
Abstract
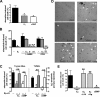
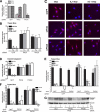
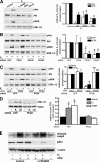
Estrogens promote bone health in part by increasing osteocyte survival, an effect that requires activation of the protein kinases Akt and ERK1/2, but the molecular mechanisms involved are only partly understood. Because estrogens increase nitric oxide (NO) synthesis and NO can have anti-apoptotic effects, we examined the role of NO/cGMP signaling in estrogen regulation of osteocyte survival. Etoposide-induced death of MLO-Y4 osteocyte-like cells, assessed by trypan blue staining, caspase-3 cleavage, and TUNEL assays, was completely prevented when cells were pre-treated with 17β-estradiol. This protective effect was mimicked when cells were pre-treated with a membrane-permeable cGMP analog and blocked by pharmacological inhibitors of NO synthase, soluble guanylate cyclase, or cGMP-dependent protein kinases (PKGs), supporting a requirement for NO/cGMP/PKG signaling downstream of 17β-estradiol. siRNA-mediated knockdown and viral reconstitution of individual PKG isoforms demonstrated that the anti-apoptotic effects of estradiol and cGMP were mediated by PKG Iα and PKG II. Akt and ERK1/2 activation by 17β-estradiol required PKG II, and cGMP mimicked the effects of estradiol on Akt and ERK, including induction of ERK nuclear translocation. cGMP induced BAD phosphorylation on several sites, and experiments with phosphorylation-deficient BAD mutants demonstrated that the anti-apoptotic effects of cGMP and 17β-estradiol required BAD phosphorylation on Ser(136) and Ser(155); these sites were targeted by Akt and PKG I, respectively, and regulate BAD interaction with Bcl-2. In conclusion, 17β-estradiol protects osteocytes against apoptosis by activating the NO/cGMP/PKG cascade; PKG II is required for estradiol-induced activation of ERK and Akt, and PKG Iα contributes to pro-survival signaling by directly phosphorylating BAD.
Figures

Similar articles
-
Nitric oxide as a mediator of estrogen effects in osteocytes.Vitam Horm. 2014;96:247-63. doi: 10.1016/B978-0-12-800254-4.00010-6. Vitam Horm. 2014. PMID: 25189390 Review.
-
Protein kinase G type-Ialpha phosphorylates the apoptosis-regulating protein Bad at serine 155 and protects against apoptosis in N1E-115 cells.Neurochem Int. 2010 Mar;56(4):546-53. doi: 10.1016/j.neuint.2009.12.017. Epub 2010 Jan 4. Neurochem Int. 2010. PMID: 20043968
-
Essential roles of the nitric oxide (no)/cGMP/protein kinase G type-Iα (PKG-Iα) signaling pathway and the atrial natriuretic peptide (ANP)/cGMP/PKG-Iα autocrine loop in promoting proliferation and cell survival of OP9 bone marrow stromal cells.J Cell Biochem. 2011 Mar;112(3):829-39. doi: 10.1002/jcb.22981. J Cell Biochem. 2011. PMID: 21328456
-
Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction.J Biol Chem. 2009 May 29;284(22):14796-808. doi: 10.1074/jbc.M806486200. Epub 2009 Mar 11. J Biol Chem. 2009. PMID: 19282289 Free PMC article.
-
Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling.Br J Pharmacol. 2007 Nov;152(6):855-69. doi: 10.1038/sj.bjp.0707409. Epub 2007 Aug 13. Br J Pharmacol. 2007. PMID: 17700722 Free PMC article. Review.
Cited by
-
Nitric Oxide Generated by Tumor-Associated Macrophages Is Responsible for Cancer Resistance to Cisplatin and Correlated With Syntaxin 4 and Acid Sphingomyelinase Inhibition.Front Immunol. 2018 May 29;9:1186. doi: 10.3389/fimmu.2018.01186. eCollection 2018. Front Immunol. 2018. PMID: 29896202 Free PMC article.
-
Effects of Caffeic Acid and Its Derivatives on Bone: A Systematic Review.Drug Des Devel Ther. 2021 Jan 22;15:259-275. doi: 10.2147/DDDT.S287280. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33519191 Free PMC article.
-
17β-Estradiol reduces mitochondrial cAMP content and cytochrome oxidase activity in a phosphodiesterase 2-dependent manner.Br J Pharmacol. 2018 Oct;175(20):3876-3890. doi: 10.1111/bph.14455. Epub 2018 Sep 8. Br J Pharmacol. 2018. PMID: 30051530 Free PMC article.
-
Prevention of Osteoporosis in the Ovariectomized Rat by Oral Administration of a Nutraceutical Combination That Stimulates Nitric Oxide Production.J Osteoporos. 2019 Jun 2;2019:1592328. doi: 10.1155/2019/1592328. eCollection 2019. J Osteoporos. 2019. PMID: 31275540 Free PMC article.
-
cGMP-Phosphodiesterase Inhibition Prevents Hypoxia-Induced Cell Death Activation in Porcine Retinal Explants.PLoS One. 2016 Nov 18;11(11):e0166717. doi: 10.1371/journal.pone.0166717. eCollection 2016. PLoS One. 2016. PMID: 27861632 Free PMC article.
References
-
- Almeida M., Han L., Martin-Millan M., Plotkin L. I., Stewart S. A., Roberson P. K., Kousteni S., O'Brien C. A., Bellido T., Parfitt A. M., Weinstein R. S., Jilka R. L., Manolagas S. C. (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 282, 27285–27297 - PMC - PubMed
-
- Tomkinson A., Reeve J., Shaw R. W., Noble B. S. (1997) The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J. Clin. Endocrinol. Metab. 82, 3128–3135 - PubMed
-
- Almeida M., Martin-Millan M., Ambrogini E., Bradsher R., 3rd, Han L., Chen X. D., Roberson P. K., Weinstein R. S., O'Brien C. A., Jilka R. L., Manolagas S. C. (2010) Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERα. J. Bone Miner. Res. 25, 769–781 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

