Preclinical evaluation of dual PI3K-mTOR inhibitors and histone deacetylase inhibitors in head and neck squamous cell carcinoma
- PMID: 22116303
- PMCID: PMC3251846
- DOI: 10.1038/bjc.2011.495
Preclinical evaluation of dual PI3K-mTOR inhibitors and histone deacetylase inhibitors in head and neck squamous cell carcinoma
Abstract
Background: We examine the potential value of a series of clinically relevant PI3K-mTOR inhibitors alone, or in combination with histone deacetylase inhibitors, in a model of head and neck squamous cell carcinoma (HNSCC).
Methods: Head and neck squamous cell carcinoma cell lines, human keratinocyte and HNSCC xenograft models were treated with histone deacetylase inhibitors (HDACIs) and new generation PI3K and dual PI3K-mTOR inhibitors either alone or in combination. Cell and tumour tissue viability and proliferation were then determined in vitro and in vivo.
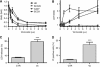
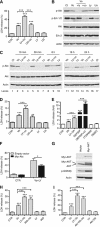
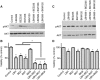
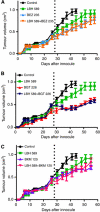
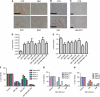
Results: Phosphatidylinositol-3-phosphate kinase, AKT and dual PI3K-mTOR inhibitors caused marked in vitro enhancement of cytotoxicity induced by HDACIs in HNSCC cancer cells. This effect correlates with AKT inhibition and is attenuated by expression of constitutively active AKT. Histone deacetylase inhibitor and phosphatidylinositol-3-phosphate kinase inhibitors (PI3KIs) inhibited tumour growth in xenograft models of HNSCC. Importantly, we observed intratumoural HDAC inhibition and PI3K inhibition as assessed by histone H3 acetylation status and phospho-AKT staining, respectively. However, we saw no evidence of improved efficacy with an HDACI/PI3KI combination.
Interpretation: That PI3K and dual PI3K-mTOR inhibitors possess antitumour effect against HNSCC in vivo.
Figures
Similar articles
-
MEK Inhibitor PD-0325901 Overcomes Resistance to PI3K/mTOR Inhibitor PF-5212384 and Potentiates Antitumor Effects in Human Head and Neck Squamous Cell Carcinoma.Clin Cancer Res. 2015 Sep 1;21(17):3946-56. doi: 10.1158/1078-0432.CCR-14-3377. Epub 2015 May 14. Clin Cancer Res. 2015. PMID: 25977343 Free PMC article.
-
PI3K/mTOR inhibitor PF-04691502 antitumor activity is enhanced with induction of wild-type TP53 in human xenograft and murine knockout models of head and neck cancer.Clin Cancer Res. 2013 Jul 15;19(14):3808-19. doi: 10.1158/1078-0432.CCR-12-2716. Epub 2013 May 2. Clin Cancer Res. 2013. PMID: 23640975 Free PMC article.
-
Superior efficacy of co-treatment with dual PI3K/mTOR inhibitor NVP-BEZ235 and pan-histone deacetylase inhibitor against human pancreatic cancer.Oncotarget. 2012 Nov;3(11):1416-27. doi: 10.18632/oncotarget.724. Oncotarget. 2012. PMID: 23232026 Free PMC article.
-
Targeting EGFR-PI3K-AKT-mTOR signaling enhances radiosensitivity in head and neck squamous cell carcinoma.Expert Opin Ther Targets. 2015 Jun;19(6):795-805. doi: 10.1517/14728222.2015.1012157. Epub 2015 Feb 5. Expert Opin Ther Targets. 2015. PMID: 25652792 Review.
-
The PI3K/Akt/mTOR axis in head and neck cancer: functions, aberrations, cross-talk, and therapies.Oral Dis. 2015 Oct;21(7):815-25. doi: 10.1111/odi.12206. Epub 2013 Dec 23. Oral Dis. 2015. PMID: 24219320 Review.
Cited by
-
Role of intratumoural heterogeneity in cancer drug resistance: molecular and clinical perspectives.EMBO Mol Med. 2012 Aug;4(8):675-84. doi: 10.1002/emmm.201101131. Epub 2012 Jun 25. EMBO Mol Med. 2012. PMID: 22733553 Free PMC article. Review.
-
Vorinostat, an HDAC inhibitor attenuates epidermoid squamous cell carcinoma growth by dampening mTOR signaling pathway in a human xenograft murine model.Toxicol Appl Pharmacol. 2013 Jan 15;266(2):233-44. doi: 10.1016/j.taap.2012.11.002. Epub 2012 Nov 9. Toxicol Appl Pharmacol. 2013. PMID: 23147569 Free PMC article.
-
mTORC1/C2 and pan-HDAC inhibitors synergistically impair breast cancer growth by convergent AKT and polysome inhibiting mechanisms.Breast Cancer Res Treat. 2014 Apr;144(2):287-298. doi: 10.1007/s10549-014-2877-y. Epub 2014 Feb 22. Breast Cancer Res Treat. 2014. PMID: 24562770 Free PMC article.
-
The anti-tumor effects of dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A on inducing autophagy in esophageal squamous cell carcinoma.J Cancer. 2018 Feb 28;9(6):987-997. doi: 10.7150/jca.22861. eCollection 2018. J Cancer. 2018. PMID: 29581778 Free PMC article.
-
Histones: Controlling Tumor Signaling Circuitry.J Carcinog Mutagen. 2013 Jul 29;1(Suppl 5):1-12. doi: 10.4172/2157-2518.S5-001. J Carcinog Mutagen. 2013. PMID: 25177526 Free PMC article.
References
-
- Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, Molinolo AA, Gutkind JS (2005) Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res 65: 9953–9961 - PubMed
-
- Amornphimoltham P, Sriuranpong V, Patel V, Benavides F, Conti CJ, Sauk J, Sausville EA, Molinolo AA, Gutkind JS (2004) Persistent activation of the Akt pathway in head and neck squamous cell carcinoma: a potential target for UCN-01. Clin Cancer Res 10: 4029–4037 - PubMed
-
- Blumenschein Jr GR, Kies MS, Papadimitrakopoulou VA, Lu C, Kumar AJ, Ricker JL, Chiao JH, Chen C, Frankel SR (2008) Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Invest New Drugs 26: 81–87 - PubMed
-
- Brinkmann H, Dahler AL, Popa C, Serewko MM, Parsons PG, Gabrielli BG, Burgess AJ, Saunders NA (2001) Histone hyperacetylation induced by histone deacetylase inhibitors is not sufficient to cause growth inhibition in human dermal fibroblasts. J Biol Chem 276: 22491–22499 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

