Human annexin A6 interacts with influenza a virus protein M2 and negatively modulates infection
- PMID: 22114333
- PMCID: PMC3264383
- DOI: 10.1128/JVI.06003-11
Human annexin A6 interacts with influenza a virus protein M2 and negatively modulates infection
Abstract
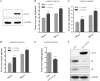
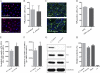
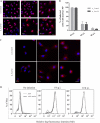
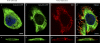
The influenza A virus M2 ion channel protein has the longest cytoplasmic tail (CT) among the three viral envelope proteins and is well conserved between different viral strains. It is accessible to the host cellular machinery after fusion with the endosomal membrane and during the trafficking, assembly, and budding processes. We hypothesized that identification of host cellular interactants of M2 CT could help us to better understand the molecular mechanisms regulating the M2-dependent stages of the virus life cycle. Using yeast two-hybrid screening with M2 CT as bait, a novel interaction with the human annexin A6 (AnxA6) protein was identified, and their physical interaction was confirmed by coimmunoprecipitation assay and a colocalization study of virus-infected human cells. We found that small interfering RNA (siRNA)-mediated knockdown of AnxA6 expression significantly increased virus production, while its overexpression could reduce the titer of virus progeny, suggesting a negative regulatory role for AnxA6 during influenza A virus infection. Further characterization revealed that AnxA6 depletion or overexpression had no effect on the early stages of the virus life cycle or on viral RNA replication but impaired the release of progeny virus, as suggested by delayed or defective budding events observed at the plasma membrane of virus-infected cells by transmission electron microscopy. Collectively, this work identifies AnxA6 as a novel cellular regulator that targets and impairs the virus budding and release stages of the influenza A virus life cycle.
Figures


Similar articles
-
Annexin A6-balanced late endosomal cholesterol controls influenza A replication and propagation.mBio. 2013 Nov 5;4(6):e00608-13. doi: 10.1128/mBio.00608-13. mBio. 2013. PMID: 24194536 Free PMC article.
-
YWHAG inhibits influenza a virus replication by suppressing the release of viral M2 protein.Front Microbiol. 2022 Jul 19;13:951009. doi: 10.3389/fmicb.2022.951009. eCollection 2022. Front Microbiol. 2022. PMID: 35928168 Free PMC article.
-
Host Cellular Protein TRAPPC6AΔ Interacts with Influenza A Virus M2 Protein and Regulates Viral Propagation by Modulating M2 Trafficking.J Virol. 2016 Dec 16;91(1):e01757-16. doi: 10.1128/JVI.01757-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795429 Free PMC article.
-
Annexin A6 is an organizer of membrane microdomains to regulate receptor localization and signalling.IUBMB Life. 2011 Nov;63(11):1009-17. doi: 10.1002/iub.540. Epub 2011 Oct 12. IUBMB Life. 2011. PMID: 21990038 Review.
-
Annexin A6 in the liver: From the endocytic compartment to cellular physiology.Biochim Biophys Acta Mol Cell Res. 2017 Jun;1864(6):933-946. doi: 10.1016/j.bbamcr.2016.10.017. Epub 2016 Oct 27. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27984093 Review.
Cited by
-
Host and virus protein interaction studies in understanding shrimp virus gene function.Indian J Virol. 2012 Sep;23(2):184-90. doi: 10.1007/s13337-012-0085-0. Epub 2012 Aug 14. Indian J Virol. 2012. PMID: 23997442 Free PMC article.
-
Viroporins: structure and biological functions.Nat Rev Microbiol. 2012 Jul 2;10(8):563-74. doi: 10.1038/nrmicro2820. Nat Rev Microbiol. 2012. PMID: 22751485 Free PMC article. Review.
-
The Role of Lipid Metabolism in Influenza A Virus Infection.Pathogens. 2021 Mar 5;10(3):303. doi: 10.3390/pathogens10030303. Pathogens. 2021. PMID: 33807642 Free PMC article. Review.
-
Human TRA2A determines influenza A virus host adaptation by regulating viral mRNA splicing.Sci Adv. 2020 Jun 19;6(25):eaaz5764. doi: 10.1126/sciadv.aaz5764. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32596447 Free PMC article.
-
Comparative proteomics reveals novel components at the plasma membrane of differentiated HepaRG cells and different distribution in hepatocyte- and biliary-like cells.PLoS One. 2013 Aug 20;8(8):e71859. doi: 10.1371/journal.pone.0071859. eCollection 2013. PLoS One. 2013. PMID: 23977166 Free PMC article.
References
-
- Barman S, Adhikary L, Kawaoka Y, Nayak DP. 2003. Influenza A virus hemagglutinin containing basolateral localization signal does not alter the apical budding of a recombinant influenza A virus in polarized MDCK cells. Virology 305:138–152 - PubMed
-
- Carlton JG, Martin-Serrano J. 2009. The ESCRT machinery: new functions in viral and cellular biology. Biochem. Soc. Trans. 37:195–199 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

