Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis
- PMID: 22108526
- PMCID: PMC3252101
- DOI: 10.1104/pp.111.189779
Changes in cell wall biomechanical properties in the xyloglucan-deficient xxt1/xxt2 mutant of Arabidopsis
Abstract
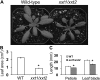
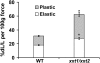
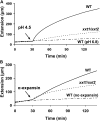
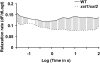
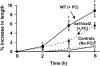
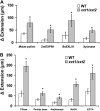
The main load-bearing network in the primary cell wall of most land plants is commonly depicted as a scaffold of cellulose microfibrils tethered by xyloglucans. However, a xyloglucan-deficient mutant (xylosyltransferase1/xylosyltransferase2 [xxt1/xxt2]) was recently developed that was smaller than the wild type but otherwise nearly normal in its development, casting doubt on xyloglucan's role in wall structure. To assess xyloglucan function in the Arabidopsis (Arabidopsis thaliana) wall, we compared the behavior of petiole cell walls from xxt1/xxt2 and wild-type plants using creep, stress relaxation, and stress/strain assays, in combination with reagents that cut or solubilize specific components of the wall matrix. Stress/strain assays showed xxt1/xxt2 walls to be more extensible than wild-type walls (supporting a reinforcing role for xyloglucan) but less extensible in creep and stress relaxation processes mediated by α-expansin. Fusicoccin-induced "acid growth" was likewise reduced in xxt1/xxt2 petioles. The results show that xyloglucan is important for wall loosening by α-expansin, and the smaller size of the xxt1/xxt2 mutant may stem from the reduced effectiveness of α-expansins in the absence of xyloglucan. Loosening agents that act on xylans and pectins elicited greater extension in creep assays of xxt1/xxt2 cell walls compared with wild-type walls, consistent with a larger mechanical role for these matrix polymers in the absence of xyloglucan. Our results illustrate the need for multiple biomechanical assays to evaluate wall properties and indicate that the common depiction of a cellulose-xyloglucan network as the major load-bearing structure is in need of revision.
Figures

Similar articles
-
Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component.Plant Cell. 2008 Jun;20(6):1519-37. doi: 10.1105/tpc.108.059873. Epub 2008 Jun 10. Plant Cell. 2008. PMID: 18544630 Free PMC article.
-
Xyloglucan Deficiency Disrupts Microtubule Stability and Cellulose Biosynthesis in Arabidopsis, Altering Cell Growth and Morphogenesis.Plant Physiol. 2016 Jan;170(1):234-49. doi: 10.1104/pp.15.01395. Epub 2015 Nov 2. Plant Physiol. 2016. PMID: 26527657 Free PMC article.
-
A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases.Plant Physiol. 2012 Apr;158(4):1933-43. doi: 10.1104/pp.111.192880. Epub 2012 Feb 23. Plant Physiol. 2012. PMID: 22362871 Free PMC article.
-
Xyloglucan and its interactions with other components of the growing cell wall.Plant Cell Physiol. 2015 Feb;56(2):180-94. doi: 10.1093/pcp/pcu204. Epub 2015 Jan 21. Plant Cell Physiol. 2015. PMID: 25613914 Review.
-
Functions of xyloglucan in plant cells.Mol Plant. 2011 Jan;4(1):17-24. doi: 10.1093/mp/ssq063. Epub 2010 Oct 13. Mol Plant. 2011. PMID: 20943810 Review.
Cited by
-
Seed coat-derived brassinosteroid signaling regulates endosperm development.Nat Commun. 2024 Oct 29;15(1):9352. doi: 10.1038/s41467-024-53671-x. Nat Commun. 2024. PMID: 39472566 Free PMC article.
-
Tensile Testing Assay for the Measurement of Tissue Stiffness in Arabidopsis Inflorescence Stem.Bio Protoc. 2019 Aug 5;9(15):e3327. doi: 10.21769/BioProtoc.3327. eCollection 2019 Aug 5. Bio Protoc. 2019. PMID: 33654834 Free PMC article.
-
Distinct cell wall architectures in seed endosperms in representatives of the Brassicaceae and Solanaceae.Plant Physiol. 2012 Nov;160(3):1551-66. doi: 10.1104/pp.112.203661. Epub 2012 Sep 6. Plant Physiol. 2012. PMID: 22961130 Free PMC article.
-
Extensins: Self-Assembly, Crosslinking, and the Role of Peroxidases.Front Plant Sci. 2021 May 14;12:664738. doi: 10.3389/fpls.2021.664738. eCollection 2021. Front Plant Sci. 2021. PMID: 34054905 Free PMC article. Review.
-
Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles.Cell Mol Life Sci. 2020 Jun;77(11):2049-2077. doi: 10.1007/s00018-019-03388-8. Epub 2019 Nov 28. Cell Mol Life Sci. 2020. PMID: 31781810 Free PMC article. Review.
References
-
- Abasolo W, Eder M, Yamauchi K, Obel N, Reinecke A, Neumetzler L, Dunlop JW, Mouille G, Pauly M, Höfte H, et al. (2009) Pectin may hinder the unfolding of xyloglucan chains during cell deformation: implications of the mechanical performance of Arabidopsis hypocotyls with pectin alterations. Mol Plant 2: 990–999 - PubMed
-
- Albersheim P, Darvill A, Roberts K, Sederoff R, Staehelin A. (2010) Plant Cell Walls. Garland Science, New York, pp 227–272
-
- Bootten TJ, Harris PJ, Melton LD, Newman RH. (2004) Solid-state 13C-NMR spectroscopy shows that the xyloglucans in the primary cell walls of mung bean (Vigna radiata L.) occur in different domains: a new model for xyloglucan-cellulose interactions in the cell wall. J Exp Bot 55: 571–583 - PubMed
-
- Bootten TJ, Harris PJ, Melton LD, Newman RH. (2009) Solid-state 13C NMR study of a composite of tobacco xyloglucan and Gluconacetobacter xylinus cellulose: molecular interactions between the component polysaccharides. Biomacromolecules 10: 2961–2967 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

