Pharmacokinetics and pharmacodynamics of VEGF-neutralizing antibodies
- PMID: 22104283
- PMCID: PMC3229549
- DOI: 10.1186/1752-0509-5-193
Pharmacokinetics and pharmacodynamics of VEGF-neutralizing antibodies
Abstract
Background: Vascular endothelial growth factor (VEGF) is a potent regulator of angiogenesis, and its role in cancer biology has been widely studied. Many cancer therapies target angiogenesis, with a focus being on VEGF-mediated signaling such as antibodies to VEGF. However, it is difficult to predict the effects of VEGF-neutralizing agents. We have developed a whole-body model of VEGF kinetics and transport under pathological conditions (in the presence of breast tumor). The model includes two major VEGF isoforms VEGF121 and VEGF165, receptors VEGFR1, VEGFR2 and co-receptors Neuropilin-1 and Neuropilin-2. We have added receptors on parenchymal cells (muscle fibers and tumor cells), and incorporated experimental data for the cell surface density of receptors on the endothelial cells, myocytes, and tumor cells. The model is applied to investigate the action of VEGF-neutralizing agents (called "anti-VEGF") in the treatment of cancer.
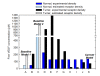
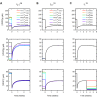
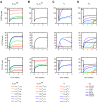
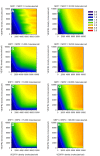
Results: Through a sensitivity study, we examine how model parameters influence the level of free VEGF in the tumor, a measure of the response to VEGF-neutralizing drugs. We investigate the effects of systemic properties such as microvascular permeability and lymphatic flow, and of drug characteristics such as the clearance rate and binding affinity. We predict that increasing microvascular permeability in the tumor above 10-5 cm/s elicits the undesired effect of increasing tumor interstitial VEGF concentration beyond even the baseline level. We also examine the impact of the tumor microenvironment, including receptor expression and internalization, as well as VEGF secretion. We find that following anti-VEGF treatment, the concentration of free VEGF in the tumor can vary between 7 and 233 pM, with a dependence on both the density of VEGF receptors and co-receptors and the rate of neuropilin internalization on tumor cells. Finally, we predict that free VEGF in the tumor is reduced following anti-VEGF treatment when VEGF121 comprises at least 25% of the VEGF secreted by tumor cells.
Conclusions: This study explores the optimal drug characteristics required for an anti-VEGF agent to have a therapeutic effect and the tumor-specific properties that influence the response to therapy. Our model provides a framework for investigating the use of VEGF-neutralizing drugs for personalized medicine treatment strategies.
Figures






Similar articles
-
Targeting neuropilin-1 to inhibit VEGF signaling in cancer: Comparison of therapeutic approaches.PLoS Comput Biol. 2006 Dec 29;2(12):e180. doi: 10.1371/journal.pcbi.0020180. Epub 2006 Nov 16. PLoS Comput Biol. 2006. PMID: 17196035 Free PMC article.
-
The presence of VEGF receptors on the luminal surface of endothelial cells affects VEGF distribution and VEGF signaling.PLoS Comput Biol. 2009 Dec;5(12):e1000622. doi: 10.1371/journal.pcbi.1000622. Epub 2009 Dec 24. PLoS Comput Biol. 2009. PMID: 20041209 Free PMC article.
-
r84, a novel therapeutic antibody against mouse and human VEGF with potent anti-tumor activity and limited toxicity induction.PLoS One. 2010 Aug 6;5(8):e12031. doi: 10.1371/journal.pone.0012031. PLoS One. 2010. PMID: 20700512 Free PMC article.
-
Vascular endothelial growth factor receptors: expression and function in solid tumors.Clin Adv Hematol Oncol. 2004 Jan;2(1):37-45. Clin Adv Hematol Oncol. 2004. PMID: 16163158 Review.
-
Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas.Pharmacol Res. 2017 Jun;120:116-132. doi: 10.1016/j.phrs.2017.03.010. Epub 2017 Mar 19. Pharmacol Res. 2017. PMID: 28330784 Review.
Cited by
-
A functional bioassay to determine the activity of anti-VEGF antibody therapy in blood of patients with cancer.Br J Cancer. 2016 Oct 11;115(8):940-948. doi: 10.1038/bjc.2016.275. Epub 2016 Aug 30. Br J Cancer. 2016. PMID: 27575850 Free PMC article.
-
Challenges in Translating from Bench to Bed-Side: Pro-Angiogenic Peptides for Ischemia Treatment.Molecules. 2019 Mar 28;24(7):1219. doi: 10.3390/molecules24071219. Molecules. 2019. PMID: 30925755 Free PMC article. Review.
-
Quantitative characterization of cellular membrane-receptor heterogeneity through statistical and computational modeling.PLoS One. 2014 May 14;9(5):e97271. doi: 10.1371/journal.pone.0097271. eCollection 2014. PLoS One. 2014. PMID: 24827582 Free PMC article.
-
A multiscale computational model predicts distribution of anti-angiogenic isoform VEGF165b in peripheral arterial disease in human and mouse.Sci Rep. 2016 Nov 17;6:37030. doi: 10.1038/srep37030. Sci Rep. 2016. PMID: 27853189 Free PMC article.
-
VEGF-121 plasma level as biomarker for response to anti-angiogenetic therapy in recurrent glioblastoma.BMC Cancer. 2018 May 10;18(1):553. doi: 10.1186/s12885-018-4442-2. BMC Cancer. 2018. PMID: 29747600 Free PMC article.
References
-
- Sung H-K, Michael IP, Nagy A. Multifaceted role of vascular endothelial growth factor signaling in adult tissue physiology: an emerging concept with clinical implications. Curr Opin Hematol. 2010;17:206–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

